Abstract
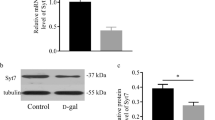
The role of sodium channel voltage-gated beta 2 (SCN2B) in brain aging is largely unknown. The present study was therefore designed to determine the role of SCN2B in brain aging by using the senescence-accelerated mice prone 8 (SAMP8), a brain senescence-accelerated animal model, together with the SCN2B transgenic mice. The results showed that SAMP8 exhibited impaired learning and memory functions, assessed by the Morris water maze test, as early as 8 months of age. The messenger RNA (mRNA) and protein expressions of SCN2B were also upregulated in the prefrontal cortex at this age. Treatment with traditional Chinese anti-aging medicine Xueshuangtong (Panax notoginseng saponins, PNS) significantly reversed the SCN2B expressions in the prefrontal cortex, resulting in improved learning and memory. Moreover, SCN2B knockdown transgenic mice were generated and bred to determine the roles of SCN2B in brain senescence. A reduction in the SCN2B level by 60.68 % resulted in improvement in the hippocampus-dependent spatial recognition memory and long-term potential (LTP) slope of field excitatory postsynaptic potential (fEPSP), followed by an upregulation of COX5A mRNA levels and downregulation of fibroblast growth factor-2 (FGF-2) mRNA expression. Together, the present findings indicated that SCN2B could play an important role in the aging-related cognitive deterioration, which is associated with the regulations of COX5A and FGF-2. These findings could provide the potential strategy of candidate target to develop antisenescence drugs for the treatment of brain aging.






Similar content being viewed by others
References
de la Torre JC (2012) A turning point for Alzheimer’s disease? Biofactors 38(2):78–83. doi:10.1002/biof.200
Bussey TJ, Wise SP, Murray EA (2001) The role of ventral and orbital PFC in conditional visuomotor learning and strategy use in rhesus monkeys (Macaca mulatta). Behav Neurosci 115(5):971–982
Otten LJ, Rugg MD (2001) Task-dependency of the neural correlates of episodic encoding as measured by fMRI. Cereb Cortex 11:1150–1160
Suzuki T, Nishiyama K, Murayama S, Yamamoto A, Sato S, Kanazawa I, Sakaki Y (1996) Regional and cellular presenilin 1 gene expression in human and rat tissues. Biochem Biophys Res Commun 219:708–713
Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA (2004) Gene regulation and DNA damage in the ageing human brain. Nature 429:883–891
Catterall WA (2000) From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26:13–25
Yu FH, Westenbroek RE, Silos-Santiago I, McCormick KA, Lawson D, Ge P, Ferriera H, Lilly J, DiStefano PS, Catterall WA, Scheuer T, Curtis R (2003) Sodium channel beta4, a new disulfide-linked auxiliary subunit with similarity to beta2. J Neurosci 23:7577–7585
Malhotra JD, Chen C, Rivolta I, Abriel H, Malhotra R, Mattei LN, Brosius FC, Kass RS, Isom LL (2001) Characterization of sodium channel alpha- and beta-subunits in rat and mouse cardiac myocytes. Circulation 103:1303–1310
Vijayaragavan K, Powell AJ, Kinghorn IJ, Chahine M (2004) Role of auxiliary beta1-, beta2-, and beta3-subunits and their interaction with Na(v)1.8 voltage-gated sodium channel. Biochem Biophys Res Commun 319:531–540
O’Malley HA, Shreiner AB, Chen GH, Huffnagle GB, Isom LL (2008) Loss of Na(+) channel beta2 subunits is neuroprotective in a mouse model of multiple sclerosis. Mol Cell Neurosci 40:143–155. doi:10.1016/j.mcn.2008.10.001
Bechtold DA, Smith KJ (2005) Sodium-mediated axonal degeneration in inflammatory demyelinating disease. J Neurol Sci 233:27–35
Waxman SG (2006) Axonal conduction and injury in multiple sclerosis: the role of sodium channels. Nat Rev Neurosci 7:932–941
Takeda T, Hosokawa M, Takeshita S, Irino M, Higuchi K, Matsushita T, Tomita Y, Yasuhira K, Hamamoto H, Shimizu K, Ishii M, Yamamuro T (1981) A new murine model of accelerated senescence. Mech Ageing 17:183–194
Nakahara H, Kanno T, Inai Y, Utsumi K, Hiramatsu M, Mori A, Packer L (1998) Mitochondrial dysfunction in the senescence accelerated mouse (SAM). Free Radic Biol Med 124:85–92
Yagi H, Katoh S, Akiguchi I, Takeda T (1988) Age-related deterioration of ability of acquisition in memory and learning in senescence accelerated mouse: SAM-P/8 as an animal model of disturbances in recent memory. Brain Res 474:86–93
Morris RGM (1984) Development of a water maze producer for studying spatial learning in the rats. J Neurosci Methods 11:47–60
Hebda-Bauer EK, Luo J, Watson SJ, Akil H (2007) Female CREB alphadelta-deficient mice show earlier age-related cognitive deficits than males. Neuroscience 150:260–272
Pouzet B, Zhang WN, Feldon J, Rawlins JN (2002) Hippocampal lesioned rats are able to learn a spatial position using non-spatial strategies. Behav Brain Res 133(2):279–291
Zhang HT, Li LY, Zou XL, Song XB, Hu YL, Feng ZT, Wang TT (2007) Immunohistochemical distribution of NGF, BDNF, NT-3, and NT-4 in adult rhesus monkey brains. J Histochem Cytochem 55(1):1–19
Hoffman AN, Krigbaum A, Ortiz JB, Mika A, Hutchinson KM, Bimonte-Nelson HA, Conrad CD (2011) Recovery after chronic stress with in spatial reference and working memory domains: correspondence with hippocampal morphology. Eur J Neurosci 34:1023–1030
Skup M, Dwornik A, Macias M, Sulejczak D, Wiater M, Czarkowska-Bauch J (2002) Long-term locomotor training up-regulates TrkB(FL) receptor-like proteins, brain-derived neurotrophic factor, and neurotrophin 4 with different topographies of expression in oligodendroglia and neurons in the spinal cord. Exp Neurol 176:289–307
Xiyang YB, Liu S, Liu J, Hao CG, Wang ZJ, Ni W, Wang XY, Wang TH (2009) Roles of platelet-derived growth factor-B expression in the ventral horn and motor cortex in the spinal cord-hemisected rhesus monkey. J Neurotrauma 26(2):275–287
Jaslove SW (1992) The integrative properties of spiny distal dendrites. Neuroscience 47:495–519
Tao WJ, Shen YS, Liu RJ, Zhao LN (2005) Anti-aging action of glycoconjugate from Gastrodia elata on mice and fruit-flies. J Biol 22:24–26
Liao QB, Liu XQ, Liu JP, Zhang YX, Nie FY, Zou K (2006) Relationship between antioxidant activity and gastrodin content of Gastrodia elata Blume. J China Three Gorges Univ Nat Sci 28:80–82
Liu W, Wu QF, Li H, Zuo J (2002) Preventing effects of PNS on SK-N-SH cell damage induced by glycoprival culture. Shanghai J Tradit Chin Med 36:46–49
Liu S, Fu Z (2005) The clinical application and the effects on immunoregulation of the Astragalus mongholicus. Chin J Basic Med Tradit Chin Med 18:203–205
Wang P, Zhang Z, Ma X, Huang Y, Liu X, Tu P, Tong T (2003) HDTIC-1 and HDTIC-2, two compounds extracted from Astragali Radix, delay replicative senescence of human diploid fibroblasts. Mech Ageing Dev 124:10–12
Zhao LF, Zheng YS, Piao HS, Zhang SY (2006) Antisenility effects of astragalus polysaccharide and total ginsenoside on senile mice. J Med Sci Yanbian Univ 29:249–251
Strazielle C, Jazi R, Verdier Y, Qian S, Lalonde R (2009) Regional brain metabolism with cytochrome c oxidase histochemistry in a PS1/A246E mouse model of autosomal dominant Alzheimer’s disease: correlations with behavior and oxidative stress. Neurochem Int 55:806–814. doi:10.1016/j.neuint.2009.08.005
Atamna H, Kumar R (2010) Protective role of methylene blue in Alzheimer’s disease via mitochondria and cytochrome c oxidase. J Alzheimers Dis 20(Suppl 2):S439–S452. doi:10.3233/JAD-2010-100414
Arnold S, Goglia F, Kadenbach B (1998) 3,5-Diiodothyronine binds to subunit Va of cytochrome-c oxidase and abolishes the allosteric inhibition of respiration by ATP. Eur J Biochem 252:325–330
Murer MG, Boissiere F, Yan Q, Hunot S, Villares J, Faucheux B, Agid Y, Hirsch E, Raisman-Vozari R (1999) An immunohistochemical study of the distribution of brain-derived neurotrophic factor in the adult human brain, with particular reference to Alzheimer’s disease. Neuroscience 88:1015–1032
Ceda GP, Dall’Aglio E, Maggio M, Lauretani F, Bandinelli S, Falzoi C, GrimaldiW CG, Corradi F, Ferrucci L, Valenti G, Hoffman AR (2005) Clinical implications of the reduced activity of the GH-IGF-I axis in older men. J Endocrinol Investig 28:96–100
Schulte-Herbrüggen O, Eckart S, Deicke U, Kühl A, Otten U, Danker-Hopfe H, Abramowski D, Staufenbiel M, Hellweg R (2008) Age-dependent time course of cerebral brain-derived neurotrophic factor, nerve growth factor, and neurotrophin-3 in APP23 transgenic mice. J Neurosci Res 86(12):2774–2783. doi:10.1002/jnr.21704
Cheng B, Mattson MP (1992) Glucose deprivation elicits neurofibrillary tangle-like antigenic changes in hippocampal neurons: prevention by NGF and bFGF. Exp Neurol 117:114–123
Kiprianova I, Schindowski K, von Bohlen und Halbach O, Krause S, Dono R, Schwaninger M, Unsicker K (2004) Enlarged infarct volume and loss of BDNF mRNA induction following brain ischemia in mice lacking FGF-2. Exp Neurol 189:252–260
Johnson-Farley NN, Patel K, Kim D, Cowen DS (2007) Interaction of FGF-2 with IGF-1 and BDNF in stimulating Akt, ERK, and neuronal survival in hippocampal cultures. Brain Res 18:40–49
Stieber A, Mourelatos Z, Gonatas NK (1996) In Alzheimer’s disease the Golgi apparatus of a population of neurons without neurofibrillary tangles is fragmented and atrophic. Am J Pathol 148:415–426
Hanneken A, Frautschy S, Galasko D, Baird A (1995) A fibroblast growth factor binding protein in human cerebral spinal fluid. Neuroreport 6:886–888
Rohe M, Synowitz M, Glass R, Paul SM, Nykjaer A, Willnow TE (2009) Brain-derived neurotrophic factor reduces amyloidogenic processing through control of SORLA gene expression. J Neurosci 29(49):15472–15478
Acknowledgments
We thank Dr. Seng Kee Leong for his valuable comments in the writing of this manuscript. This work is supported by Key Project of the Applied Basic Research Programs of Yunnan Province in China (Grant No. 2012F001), as well as the Special Fund of the Applied Basic Research Programs of Yunnan Province associated with Kunming Medical University in China (Grant No. 2013FB116).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Yan-Bin XiYang, You-Cui Wang, Lian-Feng Zhang, and Ting-Hua Wang contributed equally to this work.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Online Resource 1
(DOC 105 kb)
Online Resource 2
(GIF 63 kb)
Online Resource 3
(GIF 146 kb)
Online Resource 4
(GIF 439 kb)
Rights and permissions
About this article
Cite this article
XiYang, YB., Wang, YC., Zhao, Y. et al. Sodium Channel Voltage-Gated Beta 2 Plays a Vital Role in Brain Aging Associated with Synaptic Plasticity and Expression of COX5A and FGF-2. Mol Neurobiol 53, 955–967 (2016). https://doi.org/10.1007/s12035-014-9048-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-9048-3