Abstract
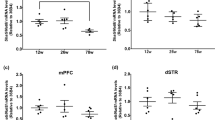
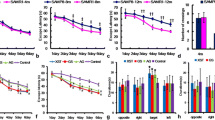
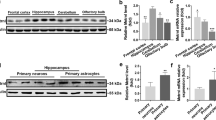
Aging is an irreversible biological process that involves oxidative stress, neuroinflammation, and apoptosis, and eventually leads to cognitive dysfunction. However, the underlying mechanisms are not fully understood. In this study, we investigated the role and potential mechanisms of Synaptotagmin-7, a calcium membrane transporter in cognitive impairment in aging mice. Our results indicated that Synaptotagmin-7 expression significantly decreased in the hippocampus of d-galactose-induced or naturally aging mice when compared with healthy controls, as detected by western blot and quantitative reverse transcriptase-polymerase chain reaction analysis. Synaptotagmin-7 overexpression in the dorsal CA1 of the hippocampus reversed long-term potentiation and improved hippocampus-dependent spatial learning in d-galactose-induced aging mice. Synaptotagmin-7 overexpression also led to fully preserved learning and memory in 6-month-old mice. Mechanistically, we demonstrated that Synaptotagmin-7 improved learning and memory by elevating the level of fEPSP and downregulating the expression of aging-related genes such as p53 and p16. The results of our study provide new insights into the role of Synaptotagmin-7 in improving neuronal function and overcoming memory impairment caused by aging, suggesting that Synaptotagmin-7 overexpression may be an innovative therapeutic strategy for treating cognitive impairment.












Similar content being viewed by others
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Petersen RC, Yaffe K (2020) Issues and questions surrounding screening for cognitive impairment in older patients. JAMA 323(8):722–724. https://doi.org/10.1001/jama.2019.22527
Iadecola C, Duering M, Hachinski V, Joutel A, Pendlebury ST, Schneider JA, Dichgans M (2019) Vascular cognitive impairment and dementia: JACC Scientific Expert Panel. J Am Coll Cardiol 73(25):3326–3344. https://doi.org/10.1016/j.jacc.2019.04.034
Wyss-Coray T (2016) Ageing, neurodegeneration and brain rejuvenation. Nature 539(7628):180–186. https://doi.org/10.1038/nature20411
Guo Y, Li H, Ke X, Deng M, Wu Z, Cai Y, Afewerky HK, Zhang X, Pei L, Lu Y (2019) Degradation of caytaxin causes learning and memory deficits via activation of DAPK1 in aging. Mol Neurobiol 56(5):3368–3379. https://doi.org/10.1007/s12035-018-1312-5
Campos PB, Paulsen BS, Rehen SK (2014) Accelerating neuronal aging in in vitro model brain disorders: a focus on reactive oxygen species. Front Aging Neurosci 6:292. https://doi.org/10.3389/fnagi.2014.00292
Bauerlein FJB, Fernandez-Busnadiego R, Baumeister W (2020) Investigating the structure of neurotoxic protein aggregates inside cells. Trends Cell Biol. https://doi.org/10.1016/j.tcb.2020.08.007
Baller EB, Kaczkurkin AN, Sotiras A, Adebimpe A, Bassett DS, Calkins ME, Chand G, Cui Z, Gur RE, Gur RC, Linn KA, Moore T, Roalf DR, Varol E, Wolf DH, Xia CH, Davatzikos C, Satterthwaite TD (2020) Neurocognitive and functional heterogeneity in depressed youth. Neuropsychopharmacology. https://doi.org/10.1038/s41386-020-00871-w
Brai E, Hummel T, Alberi L (2020) Smell, an underrated early biomarker for brain aging. Front Neurosci 14:792. https://doi.org/10.3389/fnins.2020.00792
Pei H, Ma L, Cao Y, Wang F, Li Z, Liu N, Liu M, Wei Y, Li H (2020) Traditional Chinese medicine for Alzheimer’s disease and other cognitive impairment: a review. Am J Chin Med 48(3):487–511. https://doi.org/10.1142/S0192415X20500251
Bowers MR, Reist NE (2020) Synaptotagmin: mechanisms of an electrostatic switch. Neurosci Lett 722:134834. https://doi.org/10.1016/j.neulet.2020.134834
Bowers MR, Reist NE (2020) The C2A domain of synaptotagmin is an essential component of the calcium sensor for synaptic transmission. PLoS ONE 15(2):e0228348. https://doi.org/10.1371/journal.pone.0228348
Colom-Cadena M, Spires-Jones T, Zetterberg H, Blennow K, Caggiano A, DeKosky ST, Fillit H, Harrison JE, Schneider LS, Scheltens P, de Haan W, Grundman M, van Dyck CH, Izzo NJ, Catalano SM, Working SHE, G, (2020) The clinical promise of biomarkers of synapse damage or loss in Alzheimer’s disease. Alzheimers Res Ther 12(1):21. https://doi.org/10.1186/s13195-020-00588-4
Zhang J, He J, Johnson JL, Napolitano G, Ramadass M, Rahman F, Catz SD (2019) Cross-regulation of defective endolysosome trafficking and enhanced autophagy through TFEB in UNC13D deficiency. Autophagy 15(10):1738–1756. https://doi.org/10.1080/15548627.2019.1596475
Xiao B, Li J, Fan Y, Ye M, Lv S, Xu B, Chai Y, Zhou Z, Wu M, Zhu X (2017) Downregulation of SYT7 inhibits glioblastoma growth by promoting cellular apoptosis. Mol Med Rep 16(6):9017–9022. https://doi.org/10.3892/mmr.2017.7723
Ali T, Badshah H, Kim TH, Kim MO (2015) Melatonin attenuates D-galactose-induced memory impairment, neuroinflammation and neurodegeneration via RAGE/NF-K B/JNK signaling pathway in aging mouse model. J Pineal Res 58(1):71–85. https://doi.org/10.1111/jpi.12194
Yoo DY, Kim W, Lee CH, Shin BN, Nam SM, Choi JH, Won MH, Yoon YS, Hwang IK (2012) Melatonin improves D-galactose-induced aging effects on behavior, neurogenesis, and lipid peroxidation in the mouse dentate gyrus via increasing pCREB expression. J Pineal Res 52(1):21–28. https://doi.org/10.1111/j.1600-079X.2011.00912.x
Li L, Chen B, Zhu R, Li R, Tian Y, Liu C, Jia Q, Wang L, Tang J, Zhao D, Mo F, Liu Y, Li Y, Orekhov AN, Bromme D, Zhang D, Gao S (2019) Fructus Ligustri Lucidi preserves bone quality through the regulation of gut microbiota diversity, oxidative stress, TMAO and Sirt6 levels in aging mice. Aging (Albany NY) 11(21):9348–9368. https://doi.org/10.18632/aging.102376
Huang JL, Yu C, Su M, Yang SM, Zhang F, Chen YY, Liu JY, Jiang YF, Zhong ZG, Wu DP (2019) Probucol, a “non-statin” cholesterol-lowering drug, ameliorates D-galactose induced cognitive deficits by alleviating oxidative stress via Keap1/Nrf2 signaling pathway in mice. Aging (Albany NY) 11(19):8542–8555. https://doi.org/10.18632/aging.102337
Lu J, Wu DM, Zheng YL, Hu B, Zhang ZF, Ye Q, Liu CM, Shan Q, Wang YJ (2010) Ursolic acid attenuates D-galactose-induced inflammatory response in mouse prefrontal cortex through inhibiting AGEs/RAGE/NF-kappaB pathway activation. Cereb Cortex 20(11):2540–2548. https://doi.org/10.1093/cercor/bhq002
Bo-Htay C, Palee S, Apaijai N, Chattipakorn SC, Chattipakorn N (2018) Effects of d-galactose-induced ageing on the heart and its potential interventions. J Cell Mol Med 22(3):1392–1410. https://doi.org/10.1111/jcmm.13472
Shwe T, Pratchayasakul W, Chattipakorn N, Chattipakorn SC (2018) Role of D-galactose-induced brain aging and its potential used for therapeutic interventions. Exp Gerontol 101:13–36. https://doi.org/10.1016/j.exger.2017.10.029
Hsieh HM, Wu WM, Hu ML (2009) Soy isoflavones attenuate oxidative stress and improve parameters related to aging and Alzheimer’s disease in C57BL/6J mice treated with D-galactose. Food Chem Toxicol 47(3):625–632. https://doi.org/10.1016/j.fct.2008.12.026
Offenburger SL, Ho XY, Tachie-Menson T, Coakley S, Hilliard MA, Gartner A (2018) 6-OHDA-induced dopaminergic neurodegeneration in Caenorhabditis elegans is promoted by the engulfment pathway and inhibited by the transthyretin-related protein TTR-33. PLoS Genet 14(1):e1007125. https://doi.org/10.1371/journal.pgen.1007125
Turgut NH, Mert DG, Kara H, Egilmez HR, Arslanbas E, Tepe B, Gungor H, Yilmaz N, Tuncel NB (2016) Effect of black mulberry (Morus nigra) extract treatment on cognitive impairment and oxidative stress status of D-galactose-induced aging mice. Pharm Biol 54(6):1052–1064. https://doi.org/10.3109/13880209.2015.1101476
Meng X, Chu G, Yang Z, Qiu P, Hu Y, Chen X, Peng W, Ye C, He FF, Zhang C (2016) Metformin protects neurons against oxygen-glucose deprivation/reoxygenation -induced injury by down-regulating MAD2B. Cell Physiol Biochem 40(3–4):477–485. https://doi.org/10.1159/000452562
Schurman LD, Carper MC, Moncayo LV, Ogasawara D, Richardson K, Yu L, Liu X, Poklis JL, Liu QS, Cravatt BF, Lichtman AH (2019) Diacylglycerol lipase-alpha regulates hippocampal-dependent learning and memory processes in mice. J Neurosci 39(30):5949–5965. https://doi.org/10.1523/JNEUROSCI.1353-18.2019
Zhang X, Bai L, Zhang S, Zhou X, Li Y, Bai J (2018) Trx-1 ameliorates learning and memory deficits in MPTP-induced Parkinson’s disease model in mice. Free Radic Biol Med 124:380–387. https://doi.org/10.1016/j.freeradbiomed.2018.06.029
Fernandez SP, Muzerelle A, Scotto-Lomassese S, Barik J, Gruart A, Delgado-Garcia JM, Gaspar P (2017) Constitutive and acquired serotonin deficiency alters memory and hippocampal synaptic plasticity. Neuropsychopharmacology 42(2):512–523. https://doi.org/10.1038/npp.2016.134
Orr AG, Hsiao EC, Wang MM, Ho K, Kim DH, Wang X, Guo W, Kang J, Yu GQ, Adame A, Devidze N, Dubal DB, Masliah E, Conklin BR, Mucke L (2015) Astrocytic adenosine receptor A2A and Gs-coupled signaling regulate memory. Nat Neurosci 18(3):423–434. https://doi.org/10.1038/nn.3930
Serrano ME, Bartholome O, Van den Ackerveken P, Ferrara A, Rogister B, Plenevaux A, Tirelli E (2019) Anxiety-like features and spatial memory problems as a consequence of hippocampal SV2A expression. PLoS ONE 14(6):e0217882. https://doi.org/10.1371/journal.pone.0217882
Liu JH, Wang Q, You QL, Li ZL, Hu NY, Wang Y, Jin ZL, Li SJ, Li XW, Yang JM, Zhu XH, Dai YF, Xu JP, Bai XC, Gao TM (2020) Acute EPA-induced learning and memory impairment in mice is prevented by DHA. Nat Commun 11(1):5465. https://doi.org/10.1038/s41467-020-19255-1
Bendahmane M, Morales A, Kreutzberger AJB, Schenk NA, Mohan R, Bakshi S, Philippe JM, Zhang S, Kiessling V, Tamm LK, Giovannucci DR, Jenkins PM, Anantharam A (2020) Synaptotagmin-7 enhances calcium-sensing of chromaffin cell granules and slows discharge of granule cargos. J Neurochem 154(6):598–617. https://doi.org/10.1111/jnc.14986
Bacaj T, Wu D, Burre J, Malenka RC, Liu X, Sudhof TC (2015) Synaptotagmin-1 and -7 are redundantly essential for maintaining the capacity of the readily-releasable pool of synaptic vesicles. PLoS Biol 13(10):e1002267. https://doi.org/10.1371/journal.pbio.1002267
Xie Z, Long J, Liu J, Chai Z, Kang X, Wang C (2017) Molecular mechanisms for the coupling of endocytosis to exocytosis in neurons. Front Mol Neurosci 10:47. https://doi.org/10.3389/fnmol.2017.00047
Liu X, Li C, Yang Y, Liu X, Li R, Zhang M, Yin Y, Qu Y (2019) Synaptotagmin 7 in twist-related protein 1-mediated epithelial - mesenchymal transition of non-small cell lung cancer. EBioMedicine 46:42–53. https://doi.org/10.1016/j.ebiom.2019.07.071
Chen P, Chen F, Lei J, Li Q, Zhou B (2019) Activation of the miR-34a-mediated SIRT1/mTOR signaling pathway by urolithin A attenuates D-galactose-induced brain aging in mice. Neurotherapeutics 16(4):1269–1282. https://doi.org/10.1007/s13311-019-00753-0
Zhuang K, Huang C, Leng L, Zheng H, Gao Y, Chen G, Ji Z, Sun H, Hu Y, Wu D, Shi M, Li H, Zhao Y, Zhang Y, Xue M, Bu G, Huang TY, Xu H, Zhang J (2018) Neuron-specific menin deletion leads to synaptic dysfunction and cognitive impairment by modulating p35 expression. Cell Rep 24(3):701–712. https://doi.org/10.1016/j.celrep.2018.06.055
Ren QG, Gong WG, Zhou H, Shu H, Wang YJ, Zhang ZJ (2019) Spatial training ameliorates long-term Alzheimer’s disease-like pathological deficits by reducing NLRP3 inflammasomes in PR5 mice. Neurotherapeutics 16(2):450–464. https://doi.org/10.1007/s13311-018-00698-w
Zhao W, Xu Z, Cao J, Fu Q, Wu Y, Zhang X, Long Y, Zhang X, Yang Y, Li Y, Mi W (2019) Elamipretide (SS-31) improves mitochondrial dysfunction, synaptic and memory impairment induced by lipopolysaccharide in mice. J Neuroinflammation 16(1):230. https://doi.org/10.1186/s12974-019-1627-9
Fei Z, Gao W, Xie R, Feng G, Chen X, Jiang Y (2019) Synaptotagmin-7, a binding protein of P53, inhibits the senescence and promotes the tumorigenicity of lung cancer cells. Biosci Rep 39 (2)https://doi.org/10.1042/BSR20181298
Luo Y, Yu Y, Zhang M, He H, Fan N (2020) Chronic administration of ketamine induces cognitive deterioration by restraining synaptic signaling. Mol Psychiatry. https://doi.org/10.1038/s41380-020-0793-6
Ge Y, Tian M, Liu L, Wong TP, Gong B, Wu D, Cho T, Lin S, Kast J, Lu J, Wang YT (2019) p97 regulates GluA1 homomeric AMPA receptor formation and plasma membrane expression. Nat Commun 10(1):4089. https://doi.org/10.1038/s41467-019-12096-7
Zhang T, Pang P, Fang Z, Guo Y, Li H, Li X, Tian T, Yang X, Chen W, Shu S, Tang N, Wu J, Zhu H, Pei L, Liu D, Tian Q, Wang J, Wang L, Zhu LQ, Lu Y (2018) Expression of BC1 impairs spatial learning and memory in Alzheimer’s disease via APP translation. Mol Neurobiol 55(7):6007–6020. https://doi.org/10.1007/s12035-017-0820-z
Acknowledgements
We are grateful to Dr. Anni Jiang and Dr. Yuting Zhu for language modification.
Funding
This work was supported by grants from the National Natural Science Foundation of China (grant numbers 81471490, 81671066, and 81974162).
Author information
Authors and Affiliations
Contributions
Yaru Xie and Xianfang Meng performed the experiments; Kaining Zhi analyzed the data; Xianfang Meng designed and supervised the study. All authors wrote the manuscript.
Corresponding author
Ethics declarations
Ethics approval
All experimental procedures were reviewed and approved by the Huazhong University of Science and Technology Ethics Committee for Care and Use of Laboratory Animals.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Highlights
•The expression of Syt7 was decreased in the hippocampus of aging mice.
•Syt7 overexpression in CA1 improved cognitive function of mice.
•Syt7 inhibited senescence by regulating the expression of p16 and p53.
•Syt7 is a valuable target for treating neurodegenerative diseases.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Xie, Y., Zhi, K. & Meng, X. Effects and Mechanisms of Synaptotagmin-7 in the Hippocampus on Cognitive Impairment in Aging Mice. Mol Neurobiol 58, 5756–5771 (2021). https://doi.org/10.1007/s12035-021-02528-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-021-02528-1