Abstract
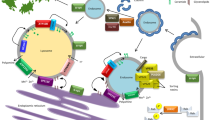
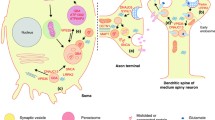
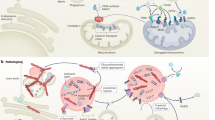
Parkinson’s disease is a common progressive neurodegenerative disorder characterized by predominant degeneration of the dopaminergic neurons in the substantia nigra pars compacta and the presence of intracellular inclusions enriched in α-synuclein, resulting in a variety motor and nonmotor symptoms. Lysosomal storage disorders are a group of disorders including Gaucher disease, Niemann-Pick disease, and neuronal ceroid lipofuscinoses caused by the defective activity of lysosomal and nonlysosomal proteins. In addition to an overlap in some clinical features between lysosomal storage disorders and Parkinson’s disease, the two disorders may be also linked pathogenically. There is growing support for the notion that mutations in genes causing lysosomal storage disorders including the glucocerebrosidase gene, the sphingomyelin phosphodiesterase 1 gene, and the NPC1 gene may increase risk for developing Parkinson’s disease. In this review, we discuss the recent advances in the genetic convergence of Parkinson’s disease and lysosomal storage disorders, shedding new light on the understanding of shared pathogenic pathways.

Similar content being viewed by others
References
Jankovic J (2011) Diagnosis and treatment of psychogenic parkinsonism. J Neurol Neurosurg Psychiatry 82(12):1300–1303
Deng H, Gao K, Jankovic J (2013) The VPS35 gene and Parkinson’s disease. Mov Disord 28(5):569–575
Seregin SS, Amalfitano A (2011) Gene therapy for lysosomal storage diseases: progress, challenges and future prospects. Curr Pharm Des 17(24):2558–2574
Gritti A (2011) Gene therapy for lysosomal storage disorders. Expert Opin Biol Ther 11(9):1153–1167
Platt FM (2014) Sphingolipid lysosomal storage disorders. Nature 510(7503):68–75
Siebert M, Sidransky E, Westbroek W (2014) Glucocerebrosidase is shaking up the synucleinopathies. Brain 137(Pt 5):1304–1322
Futerman AH, van Meer G (2004) The cell biology of lysosomal storage disorders. Nat Rev Mol Cell Biol 5(7):554–565
Schulze H, Sandhoff K (2011) Lysosomal lipid storage diseases. Cold Spring Harb Perspect Biol 3(6)
Vitner EB, Platt FM, Futerman AH (2010) Common and uncommon pathogenic cascades in lysosomal storage diseases. J Biol Chem 285(27):20423–20427
Sidransky E, Lopez G (2012) The link between the GBA gene and parkinsonism. Lancet Neurol 11(11):986–998
Wong K, Sidransky E, Verma A, Mixon T, Sandberg GD, Wakefield LK, Morrison A, Lwin A, Colegial C, Allman JM, Schiffmann R (2004) Neuropathology provides clues to the pathophysiology of Gaucher disease. Mol Genet Metab 82(3):192–207
Balducci C, Pierguidi L, Persichetti E, Parnetti L, Sbaragli M, Tassi C, Orlacchio A, Calabresi P, Beccari T, Rossi A (2007) Lysosomal hydrolases in cerebrospinal fluid from subjects with Parkinson’s disease. Mov Disord 22(10):1481–1484
Parnetti L, Balducci C, Pierguidi L, De Carlo C, Peducci M, D’Amore C, Padiglioni C, Mastrocola S, Persichetti E, Paciotti S, Bellomo G, Tambasco N, Rossi A, Beccari T, Calabresi P (2009) Cerebrospinal fluid beta-glucocerebrosidase activity is reduced in dementia with Lewy bodies. Neurobiol Dis 34(3):484–486
Foo JN, Liany H, Bei JX, Yu XQ, Liu J, Au WL, Prakash KM, Tan LC, Tan EK (2013) A rare lysosomal enzyme gene SMPD1 variant (p.R591C) associates with Parkinson’s disease. Neurobiol Aging 34(12):2890.e13-15
Gan-Or Z, Ozelius LJ, Bar-Shira A, Saunders-Pullman R, Mirelman A, Kornreich R, Gana-Weisz M, Raymond D, Rozenkrantz L, Deik A, Gurevich T, Gross SJ, Schreiber-Agus N, Giladi N, Bressman SB, Orr-Urtreger A (2013) The p.L302P mutation in the lysosomal enzyme gene SMPD1 is a risk factor for Parkinson’s disease. Neurology 80(17):1606–1610
Shachar T, Lo BC, Recchia A, Wiessner C, Raas-Rothschild A, Futerman AH (2011) Lysosomal storage disorders and Parkinson’s disease: Gaucher disease and beyond. Mov Disord 26(9):1593–1604
Goker-Alpan O, Masdeu JC, Kohn PD, Ianni A, Lopez G, Groden C, Chapman MC, Cropp B, Eisenberg DP, Maniwang ED, Davis J, Wiggs E, Sidransky E, Berman KF (2012) The neurobiology of glucocerebrosidase-associated parkinsonism: a positron emission tomography study of dopamine synthesis and regional cerebral blood flow. Brain 135(Pt 8):2440–2448
Hruska KS, LaMarca ME, Scott CR, Sidransky E (2008) Gaucher disease: mutation and polymorphism spectrum in the glucocerebrosidase gene (GBA). Hum Mutat 29(5):567–583
Inzelberg R, Hassin-Baer S, Jankovic J (2014) Genetic movement disorders in adult patients of Jewish ancestry. JAMA Neurol
Dvir H, Harel M, McCarthy AA, Toker L, Silman I, Futerman AH, Sussman JL (2003) X-ray structure of human acid-beta-glucosidase, the defective enzyme in Gaucher disease. Embo Rep 4(7):704–709
Schmitz M, Alfalah M, Aerts JM, Naim HY, Zimmer KP (2005) Impaired trafficking of mutants of lysosomal glucocerebrosidase in Gaucher’s disease. Int J Biochem Cell Biol 37(11):2310–2320
Yang NY, Lee YN, Lee HJ, Kim YS, Lee SJ (2013) Glucocerebrosidase, a new player changing the old rules in Lewy body diseases. Biol Chem 394(7):807–818
DePaolo J, Goker-Alpan O, Samaddar T, Lopez G, Sidransky E (2009) The association between mutations in the lysosomal protein glucocerebrosidase and parkinsonism. Mov Disord 24(11):1571–1578
Sorge JA, West C, Kuhl W, Treger L, Beutler E (1987) The human glucocerebrosidase gene has two functional ATG initiator codons. Am J Hum Genet 41(6):1016–1024
Brumshtein B, Wormald MR, Silman I, Futerman AH, Sussman JL (2006) Structural comparison of differently glycosylated forms of acid-beta-glucosidase, the defective enzyme in Gaucher disease. Acta Crystallogr D Biol Crystallogr 62(Pt 12):1458–1465
Jovic M, Kean MJ, Szentpetery Z, Polevoy G, Gingras AC, Brill JA, Balla T (2012) Two phosphatidylinositol 4-kinases control lysosomal delivery of the Gaucher disease enzyme, beta-glucocerebrosidase. Mol Biol Cell 23(8):1533–1545
Reczek D, Schwake M, Schroder J, Hughes H, Blanz J, Jin X, Brondyk W, Van Patten S, Edmunds T, Saftig P (2007) LIMP-2 is a receptor for lysosomal mannose-6-phosphate-independent targeting of beta-glucocerebrosidase. Cell 131(4):770–783
Goker-Alpan O, Hruska KS, Orvisky E, Kishnani PS, Stubblefield BK, Schiffmann R, Sidransky E (2005) Divergent phenotypes in Gaucher disease implicate the role of modifiers. J Med Genet 42(6):e37
Neudorfer O, Giladi N, Elstein D, Abrahamov A, Turezkite T, Aghai E, Reches A, Bembi B, Zimran A (1996) Occurrence of Parkinson’s syndrome in type I Gaucher disease. QJM 89(9):691–694
Alcalay RN, Dinur T, Quinn T, Sakanaka K, Levy O, Waters C, Fahn S, Dorovski T, Chung WK, Pauciulo M, Nichols W, Rana HQ, Balwani M, Bier L, Elstein D, Zimran A (2014) Comparison of Parkinson risk in Ashkenazi Jewish patients with Gaucher disease and GBA heterozygotes. JAMA Neurol 71(6):752–757
Goker-Alpan O, Schiffmann R, LaMarca ME, Nussbaum RL, McInerney-Leo A, Sidransky E (2004) Parkinsonism among Gaucher disease carriers. J Med Genet 41(12):937–940
Aharon-Peretz J, Rosenbaum H, Gershoni-Baruch R (2004) Mutations in the glucocerebrosidase gene and Parkinson’s disease in Ashkenazi Jews. N Engl J Med 351(19):1972–1977
Lwin A, Orvisky E, Goker-Alpan O, LaMarca ME, Sidransky E (2004) Glucocerebrosidase mutations in subjects with parkinsonism. Mol Genet Metab 81(1):70–73
Tan EK, Tong J, Fook-Chong S, Yih Y, Wong MC, Pavanni R, Zhao Y (2007) Glucocerebrosidase mutations and risk of Parkinson’s disease in Chinese patients. Arch Neurol 64(7):1056–1058
Gan-Or Z, Giladi N, Rozovski U, Shifrin C, Rosner S, Gurevich T, Bar-Shira A, Orr-Urtreger A (2008) Genotype-phenotype correlations between GBA mutations and Parkinson’s disease risk and onset. Neurology 70(24):2277–2283
Mata IF, Samii A, Schneer SH, Roberts JW, Griffith A, Leis BC, Schellenberg GD, Sidransky E, Bird TD, Leverenz JB, Tsuang D, Zabetian CP (2008) Glucocerebrosidase gene mutations: a risk factor for Lewy body disorders. Arch Neurol 65(3):379–382
Nichols WC, Pankratz N, Marek DK, Pauciulo MW, Elsaesser VE, Halter CA, Rudolph A, Wojcieszek J, Pfeiffer RF, Foroud T (2009) Mutations in GBA are associated with familial Parkinson’s disease susceptibility and age at onset. Neurology 72(4):310–316
Sidransky E, Nalls MA, Aasly JO, Aharon-Peretz J, Annesi G, Barbosa ER, Bar-Shira A, Berg D, Bras J, Brice A, Chen CM, Clark LN, Condroyer C, De Marco EV, Durr A, Eblan MJ, Fahn S, Farrer MJ, Fung HC, Gan-Or Z, Gasser T, Gershoni-Baruch R, Giladi N, Griffith A, Gurevich T, Januario C, Kropp P, Lang AE, Lee-Chen GJ, Lesage S, Marder K, Mata IF, Mirelman A, Mitsui J, Mizuta I, Nicoletti G, Oliveira C, Ottman R, Orr-Urtreger A, Pereira LV, Quattrone A, Rogaeva E, Rolfs A, Rosenbaum H, Rozenberg R, Samii A, Samaddar T, Schulte C, Sharma M, Singleton A, Spitz M, Tan EK, Tayebi N, Toda T, Troiano AR, Tsuji S, Wittstock M, Wolfsberg TG, Wu YR, Zabetian CP, Zhao Y, Ziegler SG (2009) Multicenter analysis of glucocerebrosidase mutations in Parkinson’s disease. N Engl J Med 361(17):1651–1661
Neumann J, Bras J, Deas E, O’Sullivan SS, Parkkinen L, Lachmann RH, Li A, Holton J, Guerreiro R, Paudel R, Segarane B, Singleton A, Lees A, Hardy J, Houlden H, Revesz T, Wood NW (2009) Glucocerebrosidase mutations in clinical and pathologically proven Parkinson’s disease. Brain 132(Pt 7):1783–1794
Gan-Or Z, Giladi N, Orr-Urtreger A (2009) Differential phenotype in Parkinson’s disease patients with severe versus mild GBA mutations. Brain 132(Pt 10):e125
Gonzalez-Del RML, Monroy JN, Suarez MA, Yescas GP, Boll WM, Lopez LM, Alonso VM (2013) The L444P GBA mutation is associated with early-onset Parkinson’s disease in Mexican Mestizos. Clin Genet 84(4):386–387
Ziegler SG, Eblan MJ, Gutti U, Hruska KS, Stubblefield BK, Goker-Alpan O, LaMarca ME, Sidransky E (2007) Glucocerebrosidase mutations in Chinese subjects from Taiwan with sporadic Parkinson’s disease. Mol Genet Metab 91(2):195–200
Wu YR, Chen CM, Chao CY, Ro LS, Lyu RK, Chang KH, Lee-Chen GJ (2007) Glucocerebrosidase gene mutation is a risk factor for early onset of Parkinson’s disease among Taiwanese. J Neurol Neurosurg Psychiatry 78(9):977–979
De Marco EV, Annesi G, Tarantino P, Rocca FE, Provenzano G, Civitelli D, Ciro CI, Annesi F, Carrideo S, Condino F, Nicoletti G, Messina D, Novellino F, Morelli M, Quattrone A (2008) Glucocerebrosidase gene mutations are associated with Parkinson’s disease in southern Italy. Mov Disord 23(3):460–463
Spitz M, Rozenberg R, Pereira LV, Reis BE (2008) Association between Parkinson’s disease and glucocerebrosidase mutations in Brazil. Parkinsonism Relat Disord 14(1):58–62
Kalinderi K, Bostantjopoulou S, Paisan-Ruiz C, Katsarou Z, Hardy J, Fidani L (2009) Complete screening for glucocerebrosidase mutations in Parkinson’s disease patients from Greece. Neurosci Lett 452(2):87–89
Emelyanov A, Boukina T, Yakimovskii A, Usenko T, Drosdova A, Zakharchuk A, Andoskin P, Dubina M, Schwarzman A, Pchelina S (2012) Glucocerebrosidase gene mutations are associated with Parkinson’s disease in Russia. Mov Disord 27(1):158–159
Hu FY, Xi J, Guo J, Yu LH, Liu L, He XH, Liu ZL, Zou XY, Xu YM (2010) Association of the glucocerebrosidase N370S allele with Parkinson’s disease in two separate Chinese Han populations of mainland China. Eur J Neurol 17(12):1476–1478
Lesage S, Condroyer C, Hecham N, Anheim M, Belarbi S, Lohman E, Viallet F, Pollak P, Abada M, Durr A, Tazir M, Brice A (2011) Mutations in the glucocerebrosidase gene confer a risk for Parkinson’s disease in North Africa. Neurology 76(3):301–303
Noreau A, Riviere JB, Diab S, Dion PA, Panisset M, Soland V, Jodoin N, Langlois M, Chouinard S, Dupre N, Rouleau GA (2011) Glucocerebrosidase mutations in a French-Canadian Parkinson’s disease cohort. Can J Neurol Sci 38(5):772–773
Lesage S, Anheim M, Condroyer C, Pollak P, Durif F, Dupuits C, Viallet F, Lohmann E, Corvol JC, Honore A, Rivaud S, Vidailhet M, Durr A, Brice A (2011) Large-scale screening of the Gaucher’s disease-related glucocerebrosidase gene in Europeans with Parkinson’s disease. Hum Mol Genet 20(1):202–210
Huang CL, Wu-Chou YH, Lai SC, Chang HC, Yeh TH, Weng YH, Chen RS, Huang YZ, Lu CS (2011) Contribution of glucocerebrosidase mutation in a large cohort of sporadic Parkinson’s disease in Taiwan. Eur J Neurol 18(10):1227–1232
Choi JM, Kim WC, Lyoo CH, Kang SY, Lee PH, Baik JS, Koh SB, Ma HI, Sohn YH, Lee MS, Kim YJ (2012) Association of mutations in the glucocerebrosidase gene with Parkinson’s disease in a Korean population. Neurosci Lett 514(1):12–15
Rosenbloom B, Balwani M, Bronstein JM, Kolodny E, Sathe S, Gwosdow AR, Taylor JS, Cole JA, Zimran A, Weinreb NJ (2011) The incidence of Parkinsonism in patients with type 1 Gaucher disease: data from the ICGG Gaucher Registry. Blood Cells Mol Dis 46(1):95–102
Wang C, Cai Y, Gu Z, Ma J, Zheng Z, Tang BS, Xu Y, Zhou Y, Feng T, Wang T, Chen SD, Chan P (2014) Clinical profiles of Parkinson’s disease associated with common leucine-rich repeat kinase 2 and glucocerebrosidase genetic variants in Chinese individuals. Neurobiol Aging 35(3):725.e1-6
Li Y, Sekine T, Funayama M, Li L, Yoshino H, Nishioka K, Tomiyama H, Hattori N (2014) Clinicogenetic study of GBA mutations in patients with familial Parkinson’s disease. Neurobiol Aging 35(4):935.e3-8
Toft M, Pielsticker L, Ross OA, Aasly JO, Farrer MJ (2006) Glucocerebrosidase gene mutations and Parkinson’s disease in the Norwegian population. Neurology 66(3):415–417
Goker-Alpan O, Stubblefield BK, Giasson BI, Sidransky E (2010) Glucocerebrosidase is present in alpha-synuclein inclusions in Lewy body disorders. Acta Neuropathol 120(5):641–649
Gan-Or Z, Bar-Shira A, Mirelman A, Gurevich T, Kedmi M, Giladi N, Orr-Urtreger A (2010) LRRK2 and GBA mutations differentially affect the initial presentation of Parkinson’s disease. Neurogenetics 11(1):121–125
Brockmann K, Hilker R, Pilatus U, Baudrexel S, Srulijes K, Magerkurth J, Hauser AK, Schulte C, Csoti I, Merten CD, Gasser T, Berg D, Hattingen E (2012) GBA-associated PD. Neurodegeneration, altered membrane metabolism, and lack of energy failure. Neurology 79(3):213–220
Winder-Rhodes SE, Evans JR, Ban M, Mason SL, Williams-Gray CH, Foltynie T, Duran R, Mencacci NE, Sawcer SJ, Barker RA (2013) Glucocerebrosidase mutations influence the natural history of Parkinson’s disease in a community-based incident cohort. Brain 136(Pt 2):392–399
Aharon-Peretz J, Badarny S, Rosenbaum H, Gershoni-Baruch R (2005) Mutations in the glucocerebrosidase gene and Parkinson’s disease: phenotype-genotype correlation. Neurology 65(9):1460–1461
Parkkinen L, Neumann J, O’Sullivan SS, Holton JL, Revesz T, Hardy J, Lees AJ (2011) Glucocerebrosidase mutations do not cause increased Lewy body pathology in Parkinson’s disease. Mol Genet Metab 103(4):410–412
Manning-Bog AB, Schule B, Langston JW (2009) Alpha-synuclein-glucocerebrosidase interactions in pharmacological Gaucher models: a biological link between Gaucher disease and parkinsonism. Neurotoxicology 30(6):1127–1132
Cullen V, Sardi SP, Ng J, Xu YH, Sun Y, Tomlinson JJ, Kolodziej P, Kahn I, Saftig P, Woulfe J, Rochet JC, Glicksman MA, Cheng SH, Grabowski GA, Shihabuddin LS, Schlossmacher MG (2011) Acid beta-glucosidase mutants linked to Gaucher disease, Parkinson’s disease, and Lewy body dementia alter alpha-synuclein processing. Ann Neurol 69(6):940–953
Yap TL, Gruschus JM, Velayati A, Westbroek W, Goldin E, Moaven N, Sidransky E, Lee JC (2011) Alpha-synuclein interacts with glucocerebrosidase providing a molecular link between Parkinson and Gaucher diseases. J Biol Chem 286(32):28080–28088
Sardi SP, Clarke J, Viel C, Chan M, Tamsett TJ, Treleaven CM, Bu J, Sweet L, Passini MA, Dodge JC, Yu WH, Sidman RL, Cheng SH, Shihabuddin LS (2013) Augmenting CNS glucocerebrosidase activity as a therapeutic strategy for parkinsonism and other Gaucher-related synucleinopathies. Proc Natl Acad Sci U S A 110(9):3537–3542
Xu YH, Sun Y, Ran H, Quinn B, Witte D, Grabowski GA (2011) Accumulation and distribution of alpha-synuclein and ubiquitin in the CNS of Gaucher disease mouse models. Mol Genet Metab 102(4):436–447
Goker-Alpan O, Giasson BI, Eblan MJ, Nguyen J, Hurtig HI, Lee VM, Trojanowski JQ, Sidransky E (2006) Glucocerebrosidase mutations are an important risk factor for Lewy body disorders. Neurology 67(5):908–910
Farrer MJ, Williams LN, Algom AA, Kachergus J, Hulihan MM, Ross OA, Rajput A, Papapetropoulos S, Mash DC, Dickson DW (2009) Glucosidase-beta variations and Lewy body disorders. Parkinsonism Relat Disord 15(6):414–416
Clark LN, Kartsaklis LA, Wolf GR, Dorado B, Ross BM, Kisselev S, Verbitsky M, Mejia-Santana H, Cote LJ, Andrews H, Vonsattel JP, Fahn S, Mayeux R, Honig LS, Marder K (2009) Association of glucocerebrosidase mutations with dementia with lewy bodies. Arch Neurol 66(5):578–583
Tsuang D, Leverenz JB, Lopez OL, Hamilton RL, Bennett DA, Schneider JA, Buchman AS, Larson EB, Crane PK, Kaye JA, Kramer P, Woltjer R, Kukull W, Nelson PT, Jicha GA, Neltner JH, Galasko D, Masliah E, Trojanowski JQ, Schellenberg GD, Yearout D, Huston H, Fritts-Penniman A, Mata IF, Wan JY, Edwards KL, Montine TJ, Zabetian CP (2012) GBA mutations increase risk for Lewy body disease with and without Alzheimer disease pathology. Neurology 79(19):1944–1950
Nalls MA, Duran R, Lopez G, Kurzawa-Akanbi M, McKeith IG, Chinnery PF, Morris CM, Theuns J, Crosiers D, Cras P, Engelborghs S, De Deyn PP, Van Broeckhoven C, Mann DM, Snowden J, Pickering-Brown S, Halliwell N, Davidson Y, Gibbons L, Harris J, Sheerin UM, Bras J, Hardy J, Clark L, Marder K, Honig LS, Berg D, Maetzler W, Brockmann K, Gasser T, Novellino F, Quattrone A, Annesi G, De Marco EV, Rogaeva E, Masellis M, Black SE, Bilbao JM, Foroud T, Ghetti B, Nichols WC, Pankratz N, Halliday G, Lesage S, Klebe S, Durr A, Duyckaerts C, Brice A, Giasson BI, Trojanowski JQ, Hurtig HI, Tayebi N, Landazabal C, Knight MA, Keller M, Singleton AB, Wolfsberg TG, Sidransky E (2013) A multicenter study of glucocerebrosidase mutations in dementia with Lewy bodies. JAMA Neurol 70(6):727–735
Segarane B, Li A, Paudel R, Scholz S, Neumann J, Lees A, Revesz T, Hardy J, Mathias CJ, Wood NW, Holton J, Houlden H (2009) Glucocerebrosidase mutations in 108 neuropathologically confirmed cases of multiple system atrophy. Neurology 72(13):1185–1186
Jamrozik Z, Lugowska A, Slawek J, Kwiecinski H (2010) Glucocerebrosidase mutations p.L444P and p.N370S are not associated with multisystem atrophy, progressive supranuclear palsy and corticobasal degeneration in Polish patients. J Neurol 257(3):459–460
Sun QY, Guo JF, Han WW, Zuo X, Wang L, Yao LY, Pan Q, Xia K, Yan XX, Tang BS (2013) Genetic association study of glucocerebrosidase gene L444P mutation in essential tremor and multiple system atrophy in mainland China. J Clin Neurosci 20(2):217–219
Srulijes K, Hauser AK, Guella I, Asselta R, Brockmann K, Schulte C, Solda G, Cilia R, Maetzler W, Schols L, Wenning GK, Poewe W, Barone P, Wullner U, Oertel W, Berg D, Goldwurm S, Gasser T (2013) No association of GBA mutations and multiple system atrophy. Eur J Neurol 20(4):e61–e62
Aykut A, Karaca E, Onay H, Ucar SK, Coker M, Cogulu O, Ozkinay F (2013) Analysis of the sphingomyelin phosphodiesterase 1 gene (SMPD1) in Turkish Niemann-Pick disease patients: mutation profile and description of a novel mutation. Gene 526(2):484–486
Vanier MT (2010) Niemann-Pick disease type C. Orphanet J Rare Dis 5:16
Greer WL, Riddell DC, Byers DM, Welch JP, Girouard GS, Sparrow SM, Gillan TL, Neumann PE (1997) Linkage of Niemann-Pick disease type D to the same region of human chromosome 18 as Niemann-Pick disease type C. Am J Hum Genet 61(1):139–142
Vykuntaraju KN, Lokanatha H, Shivananda (2012) Niemann-Pick disease type A presenting as unilateral tremors. Indian Pediatr 49(11):919–920
Volders P, Van Hove J, Lories RJ, Vandekerckhove P, Matthijs G, De Vos R, Vanier MT, Vincent MF, Westhovens R, Luyten FP (2002) Niemann-Pick disease type B: an unusual clinical presentation with multiple vertebral fractures. Am J Med Genet 109(1):42–51
Coleman RJ, Robb SA, Lake BD, Brett EM, Harding AE (1988) The diverse neurological features of Niemann-Pick disease type C: a report of two cases. Mov Disord 3(4):295–299
Josephs KA, Matsumoto JY, Lindor NM (2004) Heterozygous Niemann-Pick disease type C presenting with tremor. Neurology 63(11):2189–2190
Wu RM, Lin CH, Lin HI (2014) The p.L302P mutation in the lysosomal enzyme gene SMPD1 is a risk factor for Parkinson’s disease. Neurology 82(3):283
Rhein C, Tripal P, Seebahn A, Konrad A, Kramer M, Nagel C, Kemper J, Bode J, Muhle C, Gulbins E, Reichel M, Becker CM, Kornhuber J (2012) Functional implications of novel human acid sphingomyelinase splice variants. PLoS ONE 7(4):e35467
Lee CY, Tamura T, Rabah N, Lee DY, Ruel I, Hafiane A, Iatan I, Nyholt D, Laporte F, Lazure C, Wada I, Krimbou L, Genest J (2007) Carboxyl-terminal disulfide bond of acid sphingomyelinase is critical for its secretion and enzymatic function. Biochemistry-Us 46(51):14969–14978
Schuchman EH (2007) The pathogenesis and treatment of acid sphingomyelinase-deficient Niemann-Pick disease. J Inherit Metab Dis 30(5):654–663
Macauley SL, Sidman RL, Schuchman EH, Taksir T, Stewart GR (2008) Neuropathology of the acid sphingomyelinase knockout mouse model of Niemann-Pick A disease including structure-function studies associated with cerebellar Purkinje cell degeneration. Exp Neurol 214(2):181–192
Ledesma MD, Prinetti A, Sonnino S, Schuchman EH (2011) Brain pathology in Niemann Pick disease type A: insights from the acid sphingomyelinase knockout mice. J Neurochem 116(5):779–788
Kirkegaard T, Roth AG, Petersen NH, Mahalka AK, Olsen OD, Moilanen I, Zylicz A, Knudsen J, Sandhoff K, Arenz C, Kinnunen PK, Nylandsted J, Jaattela M (2010) Hsp70 stabilizes lysosomes and reverts Niemann-Pick disease-associated lysosomal pathology. Nature 463(7280):549–553
Arboleda G, Morales LC, Benitez B, Arboleda H (2009) Regulation of ceramide-induced neuronal death: cell metabolism meets neurodegeneration. Brain Res Rev 59(2):333–346
Dusek P, Jankovic J, Le W (2012) Iron dysregulation in movement disorders. Neurobiol Dis 46(1):1–18
Schneider SA, Dusek P, Hardy J, Westenberger A, Jankovic J, Bhatia KP (2013) Genetics and pathophysiology of neurodegeneration with brain iron accumulation (NBIA). Curr Neuropharmacol 11(1):59–79
Mielke MM, Maetzler W, Haughey NJ, Bandaru VV, Savica R, Deuschle C, Gasser T, Hauser AK, Graber-Sultan S, Schleicher E, Berg D, Liepelt-Scarfone I (2013) Plasma ceramide and glucosylceramide metabolism is altered in sporadic Parkinson’s disease and associated with cognitive impairment: a pilot study. PLoS ONE 8(9):e73094
Liu JP, Tang Y, Zhou S, Toh BH, McLean C, Li H (2010) Cholesterol involvement in the pathogenesis of neurodegenerative diseases. Mol Cell Neurosci 43(1):33–42
Saito Y, Suzuki K, Hulette CM, Murayama S (2004) Aberrant phosphorylation of alpha-synuclein in human Niemann-Pick type C1 disease. J Neuropathol Exp Neurol 63(4):323–328
Kluenemann HH, Nutt JG, Davis MY, Bird TD (2013) Parkinsonism syndrome in heterozygotes for Niemann-Pick C1. J Neurol Sci 335(1–2):219–220
Zech M, Nubling G, Castrop F, Jochim A, Schulte EC, Mollenhauer B, Lichtner P, Peters A, Gieger C, Marquardt T, Vanier MT, Latour P, Klunemann H, Trenkwalder C, Diehl-Schmid J, Perneczky R, Meitinger T, Oexle K, Haslinger B, Lorenzl S, Winkelmann J (2013) Niemann-pick C disease gene mutations and age-related neurodegenerative disorders. PLoS ONE 8(12):e82879
Chiba Y, Komori H, Takei S, Hasegawa-Ishii S, Kawamura N, Adachi K, Nanba E, Hosokawa M, Enokido Y, Kouchi Z, Yoshida F, Shimada A (2014) Niemann-Pick disease type C1 predominantly involving the frontotemporal region, with cortical and brainstem Lewy bodies: an autopsy case. Neuropathology 34(1):49–57
Muthane U, Chickabasaviah Y, Kaneski C, Shankar SK, Narayanappa G, Christopher R, Govindappa SS (2004) Clinical features of adult GM1 gangliosidosis: report of three Indian patients and review of 40 cases. Mov Disord 19(11):1334–1341
Roze E, Paschke E, Lopez N, Eck T, Yoshida K, Maurel-Ollivier A, Doummar D, Caillaud C, Galanaud D, Billette DVT, Vidailhet M, Roubergue A (2005) Dystonia and parkinsonism in GM1 type 3 gangliosidosis. Mov Disord 20(10):1366–1369
Schneider JS, Sendek S, Daskalakis C, Cambi F (2010) GM1 ganglioside in Parkinson’s disease: results of a five year open study. J Neurol Sci 292(1–2):45–51
Wu G, Lu ZH, Kulkarni N, Amin R, Ledeen RW (2011) Mice lacking major brain gangliosides develop parkinsonism. Neurochem Res 36(9):1706–1714
Wei J, Fujita M, Sekigawa A, Sekiyama K, Waragai M, Hashimoto M (2009) Gangliosides’ protection against lysosomal pathology of synucleinopathies. Autophagy 5(6):860–861
Martinez Z, Zhu M, Han S, Fink AL (2007) GM1 specifically interacts with alpha-synuclein and inhibits fibrillation. Biochemistry-Us 46(7):1868–1877
Inzelberg R, Korczyn AD (1994) Parkinsonism in adult-onset GM2 gangliosidosis. Mov Disord 9(3):375–377
Argov Z, Navon R (1984) Clinical and genetic variations in the syndrome of adult GM2 gangliosidosis resulting from hexosaminidase A deficiency. Ann Neurol 16(1):14–20
Suzuki K, Iseki E, Togo T, Yamaguchi A, Katsuse O, Katsuyama K, Kanzaki S, Shiozaki K, Kawanishi C, Yamashita S, Tanaka Y, Yamanaka S, Hirayasu Y (2007) Neuronal and glial accumulation of alpha- and beta-synucleins in human lipidoses. Acta Neuropathol 114(5):481–489
Bifsha P, Landry K, Ashmarina L, Durand S, Seyrantepe V, Trudel S, Quiniou C, Chemtob S, Xu Y, Gravel RA, Sladek R, Pshezhetsky AV (2007) Altered gene expression in cells from patients with lysosomal storage disorders suggests impairment of the ubiquitin pathway. Cell Death Differ 14(3):511–523
Mole SE, Williams RE (1993) Neuronal ceroid-lipofuscinoses
van Diggelen OP, Thobois S, Tilikete C, Zabot MT, Keulemans JL, van Bunderen PA, Taschner PE, Losekoot M, Voznyi YV (2001) Adult neuronal ceroid lipofuscinosis with palmitoyl-protein thioesterase deficiency: first adult-onset patients of a childhood disease. Ann Neurol 50(2):269–272
Lavrov AY, Ilyna ES, Zakharova EY, Boukina AM, Tishkanina SV (2002) The first three Russian cases of classical, late-infantile, neuronal ceroid lipofuscinosis. Eur J Paediatr Neurol 6(3):161–164
Nijssen PC, Brusse E, Leyten AC, Martin JJ, Teepen JL, Roos RA (2002) Autosomal dominant adult neuronal ceroid lipofuscinosis: parkinsonism due to both striatal and nigral dysfunction. Mov Disord 17(3):482–487
Valadares ER, Pizarro MX, Oliveira LR, Amorim RH, Pinheiro TM, Grieben U, Santos HH, Queiroz RR, Lopes GC, Godard AL (2011) Juvenile neuronal ceroid-lipofuscinosis: clinical and molecular investigation in a large family in Brazil. Arq Neuropsiquiatr 69(1):13–18
Aberg L, Liewendahl K, Nikkinen P, Autti T, Rinne JO, Santavuori P (2000) Decreased striatal dopamine transporter density in JNCL patients with parkinsonian symptoms. Neurology 54(5):1069–1074
Cullen V, Lindfors M, Ng J, Paetau A, Swinton E, Kolodziej P, Boston H, Saftig P, Woulfe J, Feany MB, Myllykangas L, Schlossmacher MG, Tyynela J (2009) Cathepsin D expression level affects alpha-synuclein processing, aggregation, and toxicity in vivo. Mol Brain 2:5
Bras J, Verloes A, Schneider SA, Mole SE, Guerreiro RJ (2012) Mutation of the parkinsonism gene ATP13A2 causes neuronal ceroid-lipofuscinosis. Hum Mol Genet 21(12):2646–2650
Schultheis PJ, Fleming SM, Clippinger AK, Lewis J, Tsunemi T, Giasson B, Dickson DW, Mazzulli JR, Bardgett ME, Haik KL, Ekhator O, Chava AK, Howard J, Gannon M, Hoffman E, Chen Y, Prasad V, Linn SC, Tamargo RJ, Westbroek W, Sidransky E, Krainc D, Shull GE (2013) Atp13a2-deficient mice exhibit neuronal ceroid lipofuscinosis, limited alpha-synuclein accumulation and age-dependent sensorimotor deficits. Hum Mol Genet 22(10):2067–2082
Nance CS, Klein CJ, Banikazemi M, Dikman SH, Phelps RG, McArthur JC, Rodriguez M, Desnick RJ (2006) Later-onset Fabry disease: an adult variant presenting with the cramp-fasciculation syndrome. Arch Neurol 63(3):453–457
Orimo S, Iwasaki T, Yoshino H, Arai M, Hiyamuta E (1994) An autopsied case of Fabry’s disease presenting with parkinsonism and cardiomegaly as a cardinal clinical manifestation. Rinsho Shinkeigaku 34(10):1003–1007
Borsini W, Giuliacci G, Torricelli F, Pelo E, Martinelli F, Scordo MR (2002) Anderson-Fabry disease with cerebrovascular complications in two Italian families. Neurol Sci 23(2):49–53
Buechner S, De Cristofaro MT, Ramat S, Borsini W (2006) Parkinsonism and Anderson Fabry’s disease: a case report. Mov Disord 21(1):103–107
Pan T, Kondo S, Le W, Jankovic J (2008) The role of autophagy-lysosome pathway in neurodegeneration associated with Parkinson’s disease. Brain 131(Pt 8):1969–1978
Lim J, Lee Y, Jung S, Youdim MB, Oh YJ (2014) Impaired autophagic flux is critically involved in drug-induced dopaminergic neuronal death. Parkinsonism Relat Disord 20(Suppl 1):S162–S166
Vogiatzi T, Xilouri M, Vekrellis K, Stefanis L (2008) Wild type alpha-synuclein is degraded by chaperone-mediated autophagy and macroautophagy in neuronal cells. J Biol Chem 283(35):23542–23556
Pan PY, Yue Z (2014) Genetic causes of Parkinson’s disease and their links to autophagy regulation. Parkinsonism Relat Disord 20(Suppl 1):S154–S157
Alvarez-Erviti L, Rodriguez-Oroz MC, Cooper JM, Caballero C, Ferrer I, Obeso JA, Schapira AH (2010) Chaperone-mediated autophagy markers in Parkinson’s disease brains. Arch Neurol 67(12):1464–1472
Sala G, Stefanoni G, Arosio A, Riva C, Melchionda L, Saracchi E, Fermi S, Brighina L, Ferrarese C (2014) Reduced expression of the chaperone-mediated autophagy carrier hsc70 protein in lymphomonocytes of patients with Parkinson’s disease. Brain Res 1546:46–52
Settembre C, Fraldi A, Rubinsztein DC, Ballabio A (2008) Lysosomal storage diseases as disorders of autophagy. Autophagy 4(1):113–114
Lieberman AP, Puertollano R, Raben N, Slaugenhaupt S, Walkley SU, Ballabio A (2012) Autophagy in lysosomal storage disorders. Autophagy 8(5):719–730
Settembre C, Fraldi A, Jahreiss L, Spampanato C, Venturi C, Medina D, de Pablo R, Tacchetti C, Rubinsztein DC, Ballabio A (2008) A block of autophagy in lysosomal storage disorders. Hum Mol Genet 17(1):119–129
Emmanouilidou E, Melachroinou K, Roumeliotis T, Garbis SD, Ntzouni M, Margaritis LH, Stefanis L, Vekrellis K (2010) Cell-produced alpha-synuclein is secreted in a calcium-dependent manner by exosomes and impacts neuronal survival. J Neurosci 30(20):6838–6851
Chan CS, Gertler TS, Surmeier DJ (2009) Calcium homeostasis, selective vulnerability and Parkinson’s disease. Trends Neurosci 32(5):249–256
Ballabio A, Gieselmann V (2009) Lysosomal disorders: from storage to cellular damage. Biochim Biophys Acta 1793(4):684–696
Lloyd-Evans E, Morgan AJ, He X, Smith DA, Elliot-Smith E, Sillence DJ, Churchill GC, Schuchman EH, Galione A, Platt FM (2008) Niemann-Pick disease type C1 is a sphingosine storage disease that causes deregulation of lysosomal calcium. Nat Med 14(11):1247–1255
Luiro K, Kopra O, Blom T, Gentile M, Mitchison HM, Hovatta I, Tornquist K, Jalanko A (2006) Batten disease (JNCL) is linked to disturbances in mitochondrial, cytoskeletal, and synaptic compartments. J Neurosci Res 84(5):1124–1138
Jenner P (2003) Oxidative stress in Parkinson’s disease. Ann Neurol 53(3):S26–S36, S36-38
Jenner P, Olanow CW (1996) Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 47(6 Suppl 3):S161–S170
Adam-Vizi V (2005) Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal 7(9–10):1140–1149
Jolly RD, Brown S, Das AM, Walkley SU (2002) Mitochondrial dysfunction in the neuronal ceroid-lipofuscinoses (Batten disease). Neurochem Int 40(6):565–571
Lucke T, Hoppner W, Schmidt E, Illsinger S, Das AM (2004) Fabry disease: reduced activities of respiratory chain enzymes with decreased levels of energy-rich phosphates in fibroblasts. Mol Genet Metab 82(1):93–97
Ruiperez V, Darios F, Davletov B (2010) Alpha-synuclein, lipids and Parkinson’s disease. Prog Lipid Res 49(4):420–428
Walkley SU (2004) Secondary accumulation of gangliosides in lysosomal storage disorders. Semin Cell Dev Biol 15(4):433–444
Chahine LM, Qiang J, Ashbridge E, Minger J, Yearout D, Horn S, Colcher A, Hurtig HI, Lee VM, Van Deerlin VM, Leverenz JB, Siderowf AD, Trojanowski JQ, Zabetian CP, Chen-Plotkin A (2013) Clinical and biochemical differences in patients having Parkinson disease with vs without GBA mutations. JAMA Neurol 70(7):852–858
Clark LN, Nicolai A, Afridi S, Harris J, Mejia-Santana H, Strug L, Cote LJ, Louis ED, Andrews H, Waters C, Ford B, Frucht S, Fahn S, Mayeux R, Ottman R, Marder K (2005) Pilot association study of the beta-glucocerebrosidase N370S allele and Parkinson’s disease in subjects of Jewish ethnicity. Mov Disord 20(1):100–103
Sato C, Morgan A, Lang AE, Salehi-Rad S, Kawarai T, Meng Y, Ray PN, Farrer LA, St GP, Rogaeva E (2005) Analysis of the glucocerebrosidase gene in Parkinson’s disease. Mov Disord 20(3):367–370
Eblan MJ, Nguyen J, Ziegler SG, Lwin A, Hanson M, Gallardo M, Weiser R, De Lucca M, Singleton A, Sidransky E (2006) Glucocerebrosidase mutations are also found in subjects with early-onset parkinsonism from Venezuela. Mov Disord 21(2):282–283
Clark LN, Ross BM, Wang Y, Mejia-Santana H, Harris J, Louis ED, Cote LJ, Andrews H, Fahn S, Waters C, Ford B, Frucht S, Ottman R, Marder K (2007) Mutations in the glucocerebrosidase gene are associated with early-onset Parkinson’s disease. Neurology 69(12):1270–1277
Bras J, Paisan-Ruiz C, Guerreiro R, Ribeiro MH, Morgadinho A, Januario C, Sidransky E, Oliveira C, Singleton A (2009) Complete screening for glucocerebrosidase mutations in Parkinson’s disease patients from Portugal. Neurobiol Aging 30(9):1515–1517
Socal MP, Bock H, Michelin-Tirelli K, Hilbig A, Saraiva-Pereira ML, Rieder CR, Jardim LB (2009) Parkinson’s disease and the heterozygous state for glucocerebrosidase mutations among Brazilians. Parkinsonism Relat Disord 15(1):76–78
Mitsui J, Mizuta I, Toyoda A, Ashida R, Takahashi Y, Goto J, Fukuda Y, Date H, Iwata A, Yamamoto M, Hattori N, Murata M, Toda T, Tsuji S (2009) Mutations for Gaucher disease confer high susceptibility to Parkinson’s disease. Arch Neurol 66(5):571–576
Nishioka K, Vilarino-Guell C, Cobb SA, Kachergus JM, Ross OA, Wider C, Gibson RA, Hentati F, Farrer MJ (2010) Glucocerebrosidase mutations are not a common risk factor for Parkinson’s disease in North Africa. Neurosci Lett 477(2):57–60
Mao XY, Burgunder JM, Zhang ZJ, An XK, Zhang JH, Yang Y, Li T, Wang YC, Chang XL, Peng R (2010) Association between GBA L444P mutation and sporadic Parkinson’s disease from Mainland China. Neurosci Lett 469(2):256–259
Sun QY, Guo JF, Wang L, Yu RH, Zuo X, Yao LY, Pan Q, Xia K, Tang BS (2010) Glucocerebrosidase gene L444P mutation is a risk factor for Parkinson’s disease in Chinese population. Mov Disord 25(8):1005–1011
Dos SA, Pestana CP, Diniz KR, Campos M, Abdalla-Carvalho CB, de Rosso AL, Pereira JS, Nicaretta DH, de Carvalho WL, Dos SJ, Santos-Reboucas CB, Pimentel MM (2010) Mutational analysis of GIGYF2, ATP13A2 and GBA genes in Brazilian patients with early-onset Parkinson’s disease. Neurosci Lett 485(2):121–124
Moraitou M, Hadjigeorgiou G, Monopolis I, Dardiotis E, Bozi M, Vassilatis D, Vilageliu L, Grinberg D, Xiromerisiou G, Stefanis L, Michelakakis H (2011) beta-Glucocerebrosidase gene mutations in two cohorts of Greek patients with sporadic Parkinson’s disease. Mol Genet Metab 104(1–2):149–152
Seto-Salvia N, Pagonabarraga J, Houlden H, Pascual-Sedano B, Dols-Icardo O, Tucci A, Paisan-Ruiz C, Campolongo A, Anton-Aguirre S, Martin I, Munoz L, Bufill E, Vilageliu L, Grinberg D, Cozar M, Blesa R, Lleo A, Hardy J, Kulisevsky J, Clarimon J (2012) Glucocerebrosidase mutations confer a greater risk of dementia during Parkinson’s disease course. Mov Disord 27(3):393–399
Guimaraes BC, Pereira AC, Rodrigues FC, Dos SA, Campos MJ, Dos SJ, Dos SF, de Rosso AL, Nicaretta DH, Pereira JS, Da SD, Della CM, Santos-Reboucas CB, Pimentel MM (2012) Glucocerebrosidase N370S and L444P mutations as risk factors for Parkinson’s disease in Brazilian patients. Parkinsonism Relat Disord 18(5):688–689
Wang Y, Liu L, Xiong J, Zhang X, Chen Z, Yu L, Chen C, Huang J, Zhang Z, Mohmed AA, Lin Z, Xiong N, Wang T (2012) Glucocerebrosidase L444P mutation confers genetic risk for Parkinson’s disease in central China. Behav Brain Funct 8:57
Zhang X, Bao QQ, Zhuang XS, Gan SR, Zhao D, Liu Y, Hu Q, Chen Y, Zhu F, Wang L, Wang N (2012) Association of common variants in the glucocerebrosidase gene with high susceptibility to Parkinson’s disease among Chinese. Chin J Physiol 55(6):398–404
Kumar KR, Ramirez A, Gobel A, Kresojevic N, Svetel M, Lohmann K, Sue CM, Rolfs A, Mazzulli JR, Alcalay RN, Krainc D, Klein C, Kostic V, Grunewald A (2013) Glucocerebrosidase mutations in a Serbian Parkinson’s disease population. Eur J Neurol 20(2):402–405
Duran R, Mencacci NE, Angeli AV, Shoai M, Deas E, Houlden H, Mehta A, Hughes D, Cox TM, Deegan P, Schapira AH, Lees AJ, Limousin P, Jarman PR, Bhatia KP, Wood NW, Hardy J, Foltynie T (2013) The glucocerobrosidase E326K variant predisposes to Parkinson’s disease, but does not cause Gaucher’s disease. Mov Disord 28(2):232–236
Bozi M, Papadimitriou D, Antonellou R, Moraitou M, Maniati M, Vassilatis DK, Papageorgiou SG, Leonardos A, Tagaris G, Malamis G, Theofilopoulos D, Kamakari S, Stamboulis E, Hadjigeorgiou GM, Athanassiadou A, Michelakakis H, Papadimitriou A, Gasser T, Stefanis L (2013) Genetic assessment of familial and early-onset Parkinson’s disease in a Greek population. Eur J Neurol
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81101339, 81271921), Research Fundation for the Doctoral Program of Higher Education of China (20110162110026), Sheng Hua Scholars Program of Central South University, China (H.D.), Construction Fundation for Key Subjects of the Third Xiangya Hospital, Central South University, China (H.D.), the Fundamental Research Funds for the Central Universities of Central South University (2014zzts360), China, the National Parkinson Foundation, USA (J.J.).
Conflict of Interest
The authors declare that there are no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Hao Deng and Xiaofei Xiu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Deng, H., Xiu, X. & Jankovic, J. Genetic Convergence of Parkinson’s Disease and Lysosomal Storage Disorders. Mol Neurobiol 51, 1554–1568 (2015). https://doi.org/10.1007/s12035-014-8832-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-014-8832-4