Abstract
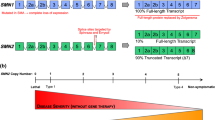
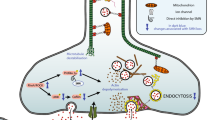
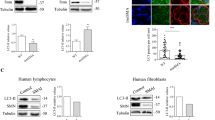
Spinal muscular atrophy (SMA) is a devastating and often fatal neurodegenerative disease that affects spinal motor neurons and leads to progressive muscle wasting and paralysis. The survival of motor neuron (SMN) gene is mutated or deleted in most forms of SMA, which results in a critical reduction in SMN protein. Motor neurons appear particularly vulnerable to reduced SMN protein levels. Therefore, understanding the functional role of SMN in protecting motor neurons from degeneration is an essential prerequisite for the design of effective therapies for SMA. To this end, there is increasing evidence indicating a key regulatory antiapoptotic role for the SMN protein that is important in motor neuron survival. The aim of this review is to highlight key findings that support an antiapoptotic role for SMN in modulating cell survival and raise possibilities for new therapeutic approaches.


Similar content being viewed by others
References
Munsat TL, Davies KE (1992) International SMA consortium meeting. (26–28 June 1992, Bonn, Germany). Neuromuscul Disord 2(5–6):423–428
Bertini E et al (2005) 134th ENMC International workshop: outcome measures and treatment of spinal muscular atrophy, 11–13 february, 2005 Naarden, The Netherlands. Neuromuscul Disord 15(11):802–816
Russman BS et al (1996) Function changes in spinal muscular atrophy II and III. The DCN/SMA Group. Neurology 47(4):973–976
Wirth B (2000) An update of the mutation spectrum of the survival motor neuron gene (SMN1) in autosomal recessive spinal muscular atrophy (SMA). Hum Mutat 15(3):228–237
Iwahashi H et al (1997) Synergistic anti-apoptotic activity between Bcl-2 and SMN implicated in spinal muscular atrophy. Nature 390(6658):413–417
Young PJ et al (2002) A direct interaction between the survival motor neuron protein and p53 and its relationship to spinal muscular atrophy. J Biol Chem 277(4):2852–2859
Gangwani L et al (2001) Spinal muscular atrophy disrupts the interaction of ZPR1 with the SMN protein. Nat Cell Biol 3(4):376–383
Soler-Botija C et al (2003) Downregulation of Bcl-2 proteins in type I spinal muscular atrophy motor neurons during fetal development. J Neuropathol Exp Neurol 62(4):420–426
Anderton RS et al (2012) Co-regulation of survival of motor neuron and Bcl-xL expression: Implications for neuroprotection in spinal muscular atrophy. Neuroscience 220:228–236
Lefebvre S et al (1995) Identification and characterization of a spinal muscular atrophy-determining gene. Cell 80(1):155–165
Rochette CF, Gilbert N, Simard LR (2001) SMN gene duplication and the emergence of the SMN2 gene occurred in distinct hominids: SMN2 is unique to Homo sapiens. Hum Genet 108(3):255–266
Boda B et al (2004) Survival motor neuron SMN1 and SMN2 gene promoters: identical sequences and differential expression in neurons and non-neuronal cells. Eur J Hum Genet 12(9):729–737
Lorson CL et al (1999) A single nucleotide in the SMN gene regulates splicing and is responsible for spinal muscular atrophy. Proc Natl Acad Sci USA 96(11):6307–6311
Cartegni L, Krainer AR (2002) Disruption of an SF2/ASF-dependent exonic splicing enhancer in SMN2 causes spinal muscular atrophy in the absence of SMN1. Nat Genet 30(4):377–384
Kashima T, Manley JL (2003) A negative element in SMN2 exon 7 inhibits splicing in spinal muscular atrophy. Nat Genet 34(4):460–463
Wang J, Dreyfuss G (2001) Characterization of functional domains of the SMN protein in vivo. J Biol Chem 276(48):45387–45393
Frugier T et al (2000) Nuclear targeting defect of SMN lacking the C-terminus in a mouse model of spinal muscular atrophy. Hum Mol Genet 9(5):849–858
Pellizzoni L, Charroux B, Dreyfuss G (1999) SMN mutants of spinal muscular atrophy patients are defective in binding to snRNP proteins. Proc Natl Acad Sci USA 96(20):11167–11172
Burnett BG et al (2009) Regulation of SMN protein stability. Mol Cell Biol 29(5):1107–1115
La Bella V et al (1998) Survival motor neuron (SMN) protein in rat is expressed as different molecular forms and is developmentally regulated. Eur J Neurosci 10(9):2913–2923
Liu Q, Dreyfuss G (1996) A novel nuclear structure containing the survival of motor neurons protein. EMBO J 15(14):3555–3565
Carvalho T et al (1999) The spinal muscular atrophy disease gene product, SMN: A link between snRNP biogenesis and the Cajal (coiled) body. J Cell Biol 147(4):715–728
Young PJ et al (2001) Nuclear gems and Cajal (coiled) bodies in fetal tissues: nucleolar distribution of the spinal muscular atrophy protein, SMN. Exp Cell Res 265(2):252–261
Hebert MD et al (2001) Coilin forms the bridge between Cajal bodies and SMN, the spinal muscular atrophy protein. Genes Dev 15(20):2720–2729
Gubitz AK, Feng W, Dreyfuss G (2004) The SMN complex. Exp Cell Res 296(1):51–56
Francis JW et al (1998) Heterogeneity of subcellular localization and electrophoretic mobility of survival motor neuron (SMN) protein in mammalian neural cells and tissues. Proc Natl Acad Sci USA 95(11):6492–6497
Pagliardini S et al (2000) Subcellular localization and axonal transport of the survival motor neuron (SMN) protein in the developing rat spinal cord. Hum Mol Genet 9(1):47–56
Broccolini A, Engel WK, Askanas V (1999) Localization of survival motor neuron protein in human apoptotic-like and regenerating muscle fibers, and neuromuscular junctions. Neuroreport 10(8):1637–1641
Battaglia G et al (1997) Expression of the SMN gene, the spinal muscular atrophy determining gene, in the mammalian central nervous system. Hum Mol Genet 6(11):1961–1971
Ruggiu M et al (2012) A role for SMN exon 7 splicing in the selective vulnerability of motor neurons in spinal muscular atrophy. Mol Cell Biol 32(1):126–138
Simic G (2008) Pathogenesis of proximal autosomal recessive spinal muscular atrophy. Acta Neuropathol 116(3):223–234
Liu Q et al (1997) The spinal muscular atrophy disease gene product, SMN, and its associated protein SIP1 are in a complex with spliceosomal snRNP proteins. Cell 90(6):1013–1021
Charroux B et al (1999) Gemin3: a novel DEAD box protein that interacts with SMN, the spinal muscular atrophy gene product, and is a component of gems. J Cell Biol 147(6):1181–1194
Charroux B et al (2000) Gemin4. A novel component of the SMN complex that is found in both gems and nucleoli. J Cell Biol 148(6):1177–1186
Baccon J et al (2002) Identification and characterization of Gemin7, a novel component of the survival of motor neuron complex. J Biol Chem 277(35):31957–31962
Gubitz AK et al (2002) Gemin5, a novel WD repeat protein component of the SMN complex that binds Sm proteins. J Biol Chem 277(7):5631–5636
Pellizzoni L, Yong J, Dreyfuss G (2002) Essential role for the SMN complex in the specificity of snRNP assembly. Science 298(5599):1775–1779
Carissimi C et al (2006) Gemin8 is a novel component of the survival motor neuron complex and functions in small nuclear ribonucleoprotein assembly. J Biol Chem 281(12):8126–8134
Raker VA et al (1999) Spliceosomal U snRNP core assembly: Sm proteins assemble onto an Sm site RNA nonanucleotide in a specific and thermodynamically stable manner. Mol Cell Biol 19(10):6554–6565
Pellizzoni L (2007) Chaperoning ribonucleoprotein biogenesis in health and disease. EMBO Rep 8(4):340–345
Kolb SJ, Battle DJ, Dreyfuss G (2007) Molecular functions of the SMN complex. J Child Neurol 22(8):990–994
Will CL, Luhrmann R (2001) Spliceosomal UsnRNP biogenesis, structure and function. Curr Opin Cell Biol 13(3):290–301
Kolb SJ, Sutton S, Schoenberg DR (2010) RNA processing defects associated with diseases of the motor neuron. Muscle Nerve 41(1):5–17
Burghes AH, Beattie CE (2009) Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nat Rev Neurosci 10(8):597–609
Lotti F et al (2012) An SMN-dependent U12 splicing event essential for motor circuit function. Cell 151(2):440–454
Zhang HL et al (2003) Active transport of the survival motor neuron protein and the role of exon-7 in cytoplasmic localization. J Neurosci 23(16):6627–6637
McWhorter ML et al (2003) Knockdown of the survival motor neuron (Smn) protein in zebrafish causes defects in motor axon outgrowth and pathfinding. J Cell Biol 162(5):919–931
Carrel TL et al (2006) Survival motor neuron function in motor axons is independent of functions required for small nuclear ribonucleoprotein biogenesis. J Neurosci 26(43):11014–11022
Fallini C et al (2011) The survival of motor neuron (SMN) protein interacts with the mRNA-binding protein HuD and regulates localization of poly(A) mRNA in primary motor neuron axons. J Neurosci 31(10):3914–3925
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26(4):239–257
Elmore S (2007) Apoptosis: a review of programmed cell death. Toxicol Pathol 35(4):495–516
Mattson MP (2000) Apoptosis in neurodegenerative disorders. Nat Rev Mol Cell Biol 1(2):120–129
Lindsten T, Zong WX, Thompson CB (2005) Defining the role of the Bcl-2 family of proteins in the nervous system. Neuroscientist 11(1):10–15
Oppenheim RW (1991) Cell death during development of the nervous system. Annu Rev Neurosci 14:453–501
Desjardins P, Ledoux S (1998) The role of apoptosis in neurodegenerative diseases. Metab Brain Dis 13(2):79–96
Martin LJ (1999) Neuronal death in amyotrophic lateral sclerosis is apoptosis: possible contribution of a programmed cell death mechanism. J Neuropathol Exp Neurol 58(5):459–471
Cleveland DW, Rothstein JD (2001) From Charcot to Lou Gehrig: deciphering selective motor neuron death in ALS. Nat Rev Neurosci 2(11):806–819
Simic G et al (2000) Ultrastructural analysis and TUNEL demonstrate motor neuron apoptosis in Werdnig–Hoffmann disease. J Neuropathol Exp Neurol 59(5):398–407
Soler-Botija C et al (2002) Neuronal death is enhanced and begins during foetal development in type I spinal muscular atrophy spinal cord. Brain 125(Pt 7):1624–1634
Tsai MS et al (2006) Abolishing Bax-dependent apoptosis shows beneficial effects on spinal muscular atrophy model mice. Mol Ther 13(6):1149–1155
Tsai MS et al (2006) Abolishing Trp53-dependent apoptosis does not benefit spinal muscular atrophy model mice. Eur J Hum Genet 14(3):372–375
Parker GC et al (2008) Survival motor neuron protein regulates apoptosis in an in vitro model of spinal muscular atrophy. Neurotox Res 13(1):39–48
Vyas S et al (2002) Involvement of survival motor neuron (SMN) protein in cell death. Hum Mol Genet 11(22):2751–2764
Wang W et al (2005) Increased susceptibility of spinal muscular atrophy fibroblasts to camptothecin-induced cell death. Mol Genet Metab 85(1):38–45
Anderton RS et al (2011) Survival of motor neuron protein over-expression prevents calpain-mediated cleavage and activation of procaspase-3 in differentiated human SH-SY5Y cells. Neuroscience 181:226–233
Kerr DA et al (2000) Survival motor neuron protein modulates neuron-specific apoptosis. Proc Natl Acad Sci USA 97(24):13312–13317
Han Z et al (1997) A sequential two-step mechanism for the production of the mature p17:p12 form of caspase-3 in vitro. J Biol Chem 272(20):13432–13436
Deveraux QL et al (1998) IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J 17(8):2215–2223
Meergans T et al (2000) The short prodomain influences caspase-3 activation in HeLa cells. Biochem J 349(Pt 1):135–140
Trulzsch B et al (2007) Knockdown of SMN by RNA interference induces apoptosis in differentiated P19 neural stem cells. Brain Res 1183:1–9
Sareen D et al (2012) Inhibition of apoptosis blocks human motor neuron cell death in a stem cell model of spinal muscular atrophy. PLoS One 7(6):e39113
Walker MP et al (2008) SMN complex localizes to the sarcomeric Z-disc and is a proteolytic target of calpain. Hum Mol Genet 17(21):3399–3410
Fuentes JL, Strayer MS, Matera AG (2010) Molecular determinants of survival motor neuron (SMN) protein cleavage by the calcium-activated protease, calpain. PLoS One 5(12):e15769
Gross A, McDonnell JM, Korsmeyer SJ (1999) BCL-2 family members and the mitochondria in apoptosis. Genes Dev 13(15):1899–1911
Burlacu A (2003) Regulation of apoptosis by Bcl-2 family proteins. J Cell Mol Med 7(3):249–257
Sato K et al (2000) Regions essential for the interaction between Bcl-2 and SMN, the spinal muscular atrophy disease gene product. Cell Death Differ 7(4):374–383
Anderson K et al (2003) Protein expression changes in spinal muscular atrophy revealed with a novel antibody array technology. Brain 126(Pt 9):2052–2064
Maheswaran S et al (1995) The WT1 gene product stabilizes p53 and inhibits p53-mediated apoptosis. Genes Dev 9(17):2143–2156
Mayo MW et al (1999) WT1 modulates apoptosis by transcriptionally upregulating the bcl-2 proto-oncogene. EMBO J 18(14):3990–4003
Helmken C et al (2003) Evidence for a modifying pathway in SMA discordant families: reduced SMN level decreases the amount of its interacting partners and Htra2-beta1. Hum Genet 114(1):11–21
Gangwani L, Flavell RA, Davis RJ (2005) ZPR1 is essential for survival and is required for localization of the survival motor neurons (SMN) protein to Cajal bodies. Mol Cell Biol 25(7):2744–2756
Doran B et al (2006) Deficiency of the zinc finger protein ZPR1 causes neurodegeneration. Proc Natl Acad Sci USA 103(19):7471–7475
Ryan KM, Phillips AC, Vousden KH (2001) Regulation and function of the p53 tumor suppressor protein. Curr Opin Cell Biol 13(3):332–337
Alarcon-Vargas D, Ronai Z (2002) p53-Mdm2—the affair that never ends. Carcinogenesis 23(4):541–547
de Rozieres S et al (2000) The loss of mdm2 induces p53-mediated apoptosis. Oncogene 19(13):1691–1697
Boise LH et al (1993) bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell 74(4):597–608
Gonzalez-Garcia M et al (1994) bcl-XL is the major bcl-x mRNA form expressed during murine development and its product localizes to mitochondria. Development 120(10):3033–3042
Kim CN et al (1997) Overexpression of Bcl-X(L) inhibits Ara-C-induced mitochondrial loss of cytochrome c and other perturbations that activate the molecular cascade of apoptosis. Cancer Res 57(15):3115–3120
Hu Y et al (1998) Bcl-XL interacts with Apaf-1 and inhibits Apaf-1-dependent caspase-9 activation. Proc Natl Acad Sci USA 95(8):4386–4391
Motoyama N et al (1995) Massive cell death of immature hematopoietic cells and neurons in Bcl-x-deficient mice. Science 267(5203):1506–1510
Tsai LK et al (2008) Restoring Bcl-x(L) levels benefits a mouse model of spinal muscular atrophy. Neurobiol Dis 31(3):361–367
Paronetto MP et al (2007) The RNA-binding protein Sam68 modulates the alternative splicing of Bcl-x. J Cell Biol 176(7):929–939
Pedrotti S et al (2010) The splicing regulator Sam68 binds to a novel exonic splicing silencer and functions in SMN2 alternative splicing in spinal muscular atrophy. EMBO J 29(7):1235–1247
Roy N et al (1995) The gene for neuronal apoptosis inhibitory protein is partially deleted in individuals with spinal muscular atrophy. Cell 80(1):167–178
Samilchuk E et al (1996) Deletion analysis of the SMN and NAIP genes in Kuwaiti patients with spinal muscular atrophy. Hum Genet 98(5):524–527
Capon F et al (1996) Deletion analysis of SMN and NAIP genes in spinal muscular atrophy Italian families. Muscle Nerve 19(3):378–380
Chang JG et al (1997) Molecular analysis of survival motor neuron (SMN) and neuronal apoptosis inhibitory protein (NAIP) genes of spinal muscular atrophy patients and their parents. Hum Genet 100(5–6):577–581
Tsai CH et al (2001) Molecular analysis of SMN, NAIP and P44 genes of SMA patients and their families. J Neurol Sci 190(1–2):35–40
Kesari A et al (2005) Study of survival of motor neuron (SMN) and neuronal apoptosis inhibitory protein (NAIP) gene deletions in SMA patients. J Neurol 252(6):667–671
Watihayati MS et al (2009) Combination of SMN2 copy number and NAIP deletion predicts disease severity in spinal muscular atrophy. Brain Dev 31(1):42–45
Maier JK et al (2002) The neuronal apoptosis inhibitory protein is a direct inhibitor of caspases 3 and 7. J Neurosci 22(6):2035–2043
Hutchison JS et al (2001) Neuronal apoptosis inhibitory protein expression after traumatic brain injury in the mouse. J Neurotrauma 18(12):1333–1347
Liston P et al (1996) Suppression of apoptosis in mammalian cells by NAIP and a related family of IAP genes. Nature 379(6563):349–353
Perrelet D et al (2000) IAP family proteins delay motoneuron cell death in vivo. Eur J Neurosci 12(6):2059–2067
Holcik M et al (2000) The hippocampal neurons of neuronal apoptosis inhibitory protein 1 (NAIP1)-deleted mice display increased vulnerability to kainic acid-induced injury. Proc Natl Acad Sci USA 97(5):2286–2290
Gotz R et al (2000) The neuronal apoptosis inhibitory protein suppresses neuronal differentiation and apoptosis in PC12 cells. Hum Mol Genet 9(17):2479–2489
Azzouz M et al (2004) Lentivector-mediated SMN replacement in a mouse model of spinal muscular atrophy. J Clin Invest 114(12):1726–1731
Foust KD et al (2010) Rescue of the spinal muscular atrophy phenotype in a mouse model by early postnatal delivery of SMN. Nat Biotechnol 28(3):271–274
Stahel RA, Zangemeister-Wittke U (2003) Antisense oligonucleotides for cancer therapy—an overview. Lung Cancer 41(Suppl 1):S81–S88
van Deutekom JC et al (2007) Local dystrophin restoration with antisense oligonucleotide PRO051. N Engl J Med 357(26):2677–2686
Cirak S et al (2011) Exon skipping and dystrophin restoration in patients with Duchenne muscular dystrophy after systemic phosphorodiamidate morpholino oligomer treatment: an open-label, phase 2, dose-escalation study. Lancet 378(9791):595–605
Hua Y et al (2007) Enhancement of SMN2 exon 7 inclusion by antisense oligonucleotides targeting the exon. PLoS Biol 5(4):e73
Passini MA et al (2011) Antisense oligonucleotides delivered to the mouse CNS ameliorate symptoms of severe spinal muscular atrophy. Sci Transl Med 3(72):72ra18
Lim ST, Airavaara M, Harvey BK (2010) Viral vectors for neurotrophic factor delivery: a gene therapy approach for neurodegenerative diseases of the CNS. Pharmacol Res 61(1):14–26
Ruixing Y, Dezhai Y, Jiaquan L (2004) Effects of cardiotrophin-1 on hemodynamics and cardiomyocyte apoptosis in rats with acute myocardial infarction. J Med Invest 51(1–2):29–37
Wen TC et al (2005) Cardiotrophin-1 protects cortical neuronal cells against free radical-induced injuries in vitro. Neurosci Lett 387(1):38–42
Bordet T et al (2001) Protective effects of cardiotrophin-1 adenoviral gene transfer on neuromuscular degeneration in transgenic ALS mice. Hum Mol Genet 10(18):1925–1933
Lesbordes JC et al (2003) Therapeutic benefits of cardiotrophin-1 gene transfer in a mouse model of spinal muscular atrophy. Hum Mol Genet 12(11):1233–1239
Peng H et al (2010) Caspase inhibition by cardiotrophin-1 prevents neuronal death in vivo and in vitro. J Neurosci Res 88(5):1041–1051
Ozdinler PH, Macklis JD (2006) IGF-I specifically enhances axon outgrowth of corticospinal motor neurons. Nat Neurosci 9(11):1371–1381
Palazzolo I et al (2009) Overexpression of IGF-1 in muscle attenuates disease in a mouse model of spinal and bulbar muscular atrophy. Neuron 63(3):316–328
Kaspar BK et al (2003) Retrograde viral delivery of IGF-1 prolongs survival in a mouse ALS model. Science 301(5634):839–842
Bosch-Marce M et al (2011) Increased IGF-1 in muscle modulates the phenotype of severe SMA mice. Hum Mol Genet 20(9):1844–1853
Passini MA et al (2010) CNS-targeted gene therapy improves survival and motor function in a mouse model of spinal muscular atrophy. J Clin Invest 120(4):1253–1264
Dominguez E et al (2011) Intravenous scAAV9 delivery of a codon-optimized SMN1 sequence rescues SMA mice. Hum Mol Genet 20(4):681–693
Porensky PN et al (2012) A single administration of morpholino antisense oligomer rescues spinal muscular atrophy in mouse. Hum Mol Genet 21(7):1625–1638
Tsai LK et al (2012) IGF-1 delivery to CNS attenuates motor neuron cell death but does not improve motor function in type III SMA mice. Neurobiol Dis 45(1):272–279
Shababi M, Glascock J, Lorson CL (2011) Combination of SMN trans-splicing and a neurotrophic factor increases the life span and body mass in a severe model of spinal muscular atrophy. Hum Gene Ther 22(2):135–144
Simic G et al (2008) Abnormal motoneuron migration, differentiation, and axon outgrowth in spinal muscular atrophy. Acta Neuropathol 115(3):313–326
Garcera A et al (2011) A new model to study spinal muscular atrophy: neurite degeneration and cell death is counteracted by BCL-X(L) Overexpression in motoneurons. Neurobiol Dis 42(3):415–426
Farooq F et al (2011) Prolactin increases SMN expression and survival in a mouse model of severe spinal muscular atrophy via the STAT5 pathway. J Clin Invest 121(8):3042–3050
Makhortova NR et al (2011) A screen for regulators of survival of motor neuron protein levels. Nat Chem Biol 7(8):544–552
Ting CH et al (2007) Stat5 constitutive activation rescues defects in spinal muscular atrophy. Hum Mol Genet 16(5):499–514
Brines ML et al (2000) Erythropoietin crosses the blood–brain barrier to protect against experimental brain injury. Proc Natl Acad Sci USA 97(19):10526–10531
Minnerup J et al (2009) The efficacy of erythropoietin and its analogues in animal stroke models: a meta-analysis. Stroke 40(9):3113–3120
Kondo A et al (2009) Erythropoietin exerts anti-epileptic effects with the suppression of aberrant new cell formation in the dentate gyrus and upregulation of neuropeptide Y in seizure model of rats. Brain Res 1296:127–136
Leist M et al (2004) Derivatives of erythropoietin that are tissue protective but not erythropoietic. Science 305(5681):239–242
Grunfeld JF et al (2007) Erythropoietin delays disease onset in an amyotrophic lateral sclerosis model. Exp Neurol 204(1):260–263
Thomson JA et al (1998) Embryonic stem cell lines derived from human blastocysts. Science 282(5391):1145–1147
Corti S et al (2010) Embryonic stem cell-derived neural stem cells improve spinal muscular atrophy phenotype in mice. Brain 133(Pt 2):465–481
Ebert AD et al (2009) Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature 457(7227):277–280
Chang JG et al (2001) Treatment of spinal muscular atrophy by sodium butyrate. Proc Natl Acad Sci USA 98(17):9808–9813
Brichta L et al (2003) Valproic acid increases the SMN2 protein level: a well-known drug as a potential therapy for spinal muscular atrophy. Hum Mol Genet 12(19):2481–2489
Andreassi C et al (2004) Phenylbutyrate increases SMN expression in vitro: relevance for treatment of spinal muscular atrophy. Eur J Hum Genet 12(1):59–65
Sumner CJ et al (2003) Valproic acid increases SMN levels in spinal muscular atrophy patient cells. Ann Neurol 54(5):647–654
Narver HL et al (2008) Sustained improvement of spinal muscular atrophy mice treated with trichostatin A plus nutrition. Ann Neurol 64(4):465–470
Grierson AJ, Shaw CE, Miller CC (2001) Androgen induced cell death in SHSY5Y neuroblastoma cells expressing wild-type and spinal bulbar muscular atrophy mutant androgen receptors. Biochim Biophys Acta 1536(1):13–20
Mercuri E et al (2007) Randomized, double-blind, placebo-controlled trial of phenylbutyrate in spinal muscular atrophy. Neurology 68(1):51–55
Swoboda KJ et al (2010) SMA CARNI-VAL trial part I: double-blind, randomized, placebo-controlled trial of L-carnitine and valproic acid in spinal muscular atrophy. PLoS One 5(8):e12140
Kissel JT et al (2011) SMA CARNIVAL TRIAL PART II: a prospective, single-armed trial of L-carnitine and valproic acid in ambulatory children with spinal muscular atrophy. PLoS One 6(7):e21296
Grzeschik SM et al (2005) Hydroxyurea enhances SMN2 gene expression in spinal muscular atrophy cells. Ann Neurol 58(2):194–202
Chen TH et al (2010) Randomized, double-blind, placebo-controlled trial of hydroxyurea in spinal muscular atrophy. Neurology 75(24):2190–2197
Bevan AK et al (2011) Systemic gene delivery in large species for targeting spinal cord, brain, and peripheral tissues for pediatric disorders. Mol Ther 19(11):1971–1980
Miller RG et al (2001) A placebo-controlled trial of gabapentin in spinal muscular atrophy. J Neurol Sci 191(1–2):127–131
ALS CNTF Treatment Study Group (1996) A double-blind placebo-controlled clinical trial of subcutaneous recombinant human ciliary neurotrophic factor (rHCNTF) in amyotrophic lateral sclerosis. Neurology 46:1244–1249
Kasarkis E (1999) A controlled trial of recombinant methionyl human BDNF in 1097 ALS: the BDNF Study Group (Phase III). Neurology 52(7):1427–33
Beck M et al (2005) Autonomic dysfunction in ALS: a preliminary study on the effects of intrathecal BDNF. Amyotroph Lateral Scler Other Motor Neuron Disord 6(2):100–103
Sorenson EJ et al (2008) Subcutaneous IGF-1 is not beneficial in 2-year ALS trial. Neurology 71(22):1770–1775
Clevenger CV, Medaglia MV (1994) The protein tyrosine kinase P59fyn is associated with prolactin (PRL) receptor and is activated by PRL stimulation of T-lymphocytes. Mol Endocrinol 8(6):674–681
Acknowledgments
These studies were supported by the Neuromuscular Foundation and Muscular Dystrophy Association of Western Australia.
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Anderton, R.S., Meloni, B.P., Mastaglia, F.L. et al. Spinal Muscular Atrophy and the Antiapoptotic Role of Survival of Motor Neuron (SMN) Protein. Mol Neurobiol 47, 821–832 (2013). https://doi.org/10.1007/s12035-013-8399-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-013-8399-5