Abstract
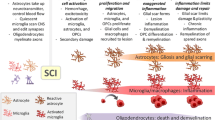
Reactive astrogliosis is a pathologic hallmark of spinal cord injury (SCI). It is characterised by profound morphological, molecular, and functional changes in astrocytes that occur within hours of SCI and evolves as time elapses after injury. Astrogliosis is a defense mechanism to minimize and repair the initial damage but eventually leads to some detrimental effects. Reactive astrocytes secrete a plethora of both growth promoting and inhibitory factors after SCI. However, the production of inhibitory components surpasses the growth stimulating factors, thus, causing inhibitory effects. In severe cases of injury, astrogliosis results in the formation of irreversible glial scarring that acts as regeneration barrier due to the expression of inhibitory components such as chondroitin sulfate proteoglycans. Scar formation was therefore recognized from a negative perspective for many years. Accumulating evidence from pharmacological and genetic studies now signifies the importance of astrogliosis and its timing for spinal cord repair. These studies have advanced our knowledge regarding signaling pathways and molecular mediators, which trigger and modulate reactive astrocytes and scar formation. In this review, we discuss the recent advances in this field. We also review therapeutic strategies that have been developed to target astrocytes reactivity and glial scaring in the environment of SCI. Astrocytes play pivotal roles in governing SCI mechanisms, and it is therefore crucial to understand how their activities can be targeted efficiently to harness their potential for repair and regeneration after SCI.

Similar content being viewed by others
Abbreviations
- BBB:
-
Blood–brain barrier
- BSB:
-
Blood–spinal barrier
- BMPs:
-
Bone morphogenetic proteins
- ChABC:
-
Chondroitin sulfate proteoglycans
- CSPGs:
-
Chondroitin sulfate proteoglycans
- CNS:
-
Central nervous system
- Eph:
-
Ephrin
- ECM:
-
Extracellular matrix
- GFAP:
-
Glial fibrillary acidic proteins
- GAG:
-
Glycosaminoglycans
- IL:
-
Interleukin
- NPCs:
-
Neural precursor cells
- OPCs:
-
Oligodendrocyte precursor cells
- RPTP:
-
Receptor protein tyrosine phosphatases
- SCI:
-
Spinal cord injury
- Stat3:
-
Signal transducers and activators of transcription3
- TGF:
-
Transforming growth factors
- TNF-α:
-
Tumor necrosis factor-alpha
- XT:
-
Xylosyltransferase
References
Sofroniew MV, Vinters HV (2010) Astrocytes: biology and pathology. Acta Neuropathol 119:7–35
Seifert G, Schilling K, Steinhauser C (2006) Astrocyte dysfunction in neurological disorders: a molecular perspective. Nat Rev Neurosci 7:194–206
Fitch MT, Silver J (1997) Glial cell extracellular matrix: boundaries for axon growth in development and regeneration. Cell Tissue Res 290:379–384
Fitch MT, Silver J (2008) CNS injury, glial scars, and inflammation: inhibitory extracellular matrices and regeneration failure. Exp Neurol 209:294–301
Silver J, Miller JH (2004) Regeneration beyond the glial scar. Nat Rev Neurosci 5:146–156
Fitch MT, Silver J (2008) CNS injury, glial scars, and inflammation: Inhibitory extracellular matrices and regeneration failure. Exp Neurol 209:294–301
Herrmann JE, Imura T, Song B, Qi J, Ao Y et al (2008) STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci 28:7231–7243
Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32:638–647
Sofroniew MV (2005) Reactive astrocytes in neural repair and protection. Neuroscientist 11:400–407
Renault-Mihara F, Katoh H, Ikegami T, Iwanami A, Mukaino M et al (2011) Beneficial compaction of spinal cord lesion by migrating astrocytes through glycogen synthase kinase-3 inhibition. EMBO Mol Med 3:682–696
Takamiya Y, Kohsaka S, Toya S, Otani M, Tsukada Y (1988) Immunohistochemical studies on the proliferation of reactive astrocytes and the expression of cytoskeletal proteins following brain injury in rats. Brain Res 466:201–210
Renault-Mihara F, Okada S, Shibata S, Nakamura M, Toyama Y et al (2008) Spinal cord injury: emerging beneficial role of reactive astrocytes’ migration. Int J Biochem Cell Biol 40:1649–1653
Bush TG, Puvanachandra N, Horner CH, Polito A, Ostenfeld T et al (1999) Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron 23:297–308
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB et al (2004) Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci 24:2143–2155
Fawcett JW (1997) Astrocytic and neuronal factors affecting axon regeneration in the damaged central nervous system. Cell Tissue Res 290:371–377
Fawcett JW, Asher RA (1999) The glial scar and central nervous system repair. Brain Res Bull 49:377–391
Busch SA, Silver J (2007) The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol 17:120–127
Bradbury EJ, Moon LD, Popat RJ, King VR, Bennett GS et al (2002) Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature 416:636–640
Fisher D, Xing B, Dill J, Li H, Hoang HH et al (2011) Leukocyte common antigen-related phosphatase is a functional receptor for chondroitin sulfate proteoglycan axon growth inhibitors. J Neurosci 31:14051–14066
Shen Y, Tenney AP, Busch SA, Horn KP, Cuascut FX et al (2009) PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science 326:592–596
Escartin C, Bonvento G (2008) Targeted activation of astrocytes: a potential neuroprotective strategy. Mol Neurobiol 38:231–241
Kang W, Hebert JM (2011) Signaling pathways in reactive astrocytes, a genetic perspective. Mol Neurobiol 43:147–154
Bushong EA, Martone ME, Ellisman MH (2004) Maturation of astrocyte morphology and the establishment of astrocyte domains during postnatal hippocampal development. Int J Dev Neurosci 22:73–86
Halassa MM, Fellin T, Takano H, Dong JH, Haydon PG (2007) Synaptic islands defined by the territory of a single astrocyte. J Neurosci 27:6473–6477
Bushong EA, Martone ME, Jones YZ, Ellisman MH (2002) Protoplasmic astrocytes in CA1 stratum radiatum occupy separate anatomical domains. J Neurosci 22:183–192
Santello M, Volterra A (2009) Synaptic modulation by astrocytes via Ca2+-dependent glutamate release. Neuroscience 158:253–259
Jourdain P, Bergersen LH, Bhaukaurally K, Bezzi P, Santello M et al (2007) Glutamate exocytosis from astrocytes controls synaptic strength. Nat Neurosci 10:331–339
Dong Y, Benveniste EN (2001) Immune function of astrocytes. Glia 36:180–190
Nicoll JA, Weller RO (2003) A new role for astrocytes: beta-amyloid homeostasis and degradation. Trends Mol Med 9:281–282
Jacobson M (1991) Developmental neurobiology. Plenum, New York
Sattler R, Rothstein JD (2006) Regulation and dysregulation of glutamate transporters. Handb Exp Pharmacol (175):277–303
Risau W, Wolburg H (1990) Development of the blood–brain barrier. Trends Neurosci 13:174–178
Wolburg H, Noell S, Mack A, Wolburg-Buchholz K, Fallier-Becker P (2009) Brain endothelial cells and the glio-vascular complex. Cell Tissue Res 335:75–96
Abbott NJ (2002) Astrocyte-endothelial interactions and blood–brain barrier permeability. J Anat 200:629–638
Wolburg HR, Risau W (1995) In: Kettenmann H, Ransom BR (eds) Formation of the blood–brain-barrier. Oxford University Press, New York
Halassa MM, Fellin T, Haydon PG (2007) The tripartite synapse: roles for gliotransmission in health and disease. Trends Mol Med 13:54–63
Perea G, Navarrete M, Araque A (2009) Tripartite synapses: astrocytes process and control synaptic information. Trends Neurosci 32:421–431
Iadecola C, Nedergaard M (2007) Glial regulation of the cerebral microvasculature. Nat Neurosci 10:1369–1376
Simard M, Nedergaard M (2004) The neurobiology of glia in the context of water and ion homeostasis. Neuroscience 129:877–896
Zador Z, Stiver S, Wang V, Manley GT (2009) Role of aquaporin-4 in cerebral edema and stroke. Handb Exp Pharmacol (190):159–170
Escartin C, Pierre K, Colin A, Brouillet E, Delzescaux T et al (2007) Activation of astrocytes by CNTF induces metabolic plasticity and increases resistance to metabolic insults. J Neurosci 27:7094–7104
Pellerin L, Bouzier-Sore AK, Aubert A, Serres S, Merle M et al (2007) Activity-dependent regulation of energy metabolism by astrocytes: an update. Glia 55:1251–1262
Takata N, Mishima T, Hisatsune C, Nagai T, Ebisui E et al (2011) Astrocyte calcium signaling transforms cholinergic modulation to cortical plasticity in vivo. J Neurosci 31:18155–18165
Qian X, Shen Q, Goderie SK, He W, Capela A et al (2000) Timing of CNS cell generation: a programmed sequence of neuron and glial cell production from isolated murine cortical stem cells. Neuron 28:69–80
Bozoyan L, Khlghatyan J, Saghatelyan A (2012) Astrocytes control the development of the migration-promoting vasculature scaffold in the postnatal brain via VEGF signaling. J Neurosci 32:1687–1704
Powell EM, Geller HM (1999) Dissection of astrocyte-mediated cues in neuronal guidance and process extension. Glia 26:73–83
Ullian EM, Sapperstein SK, Christopherson KS, Barres BA (2001) Control of synapse number by glia. Science 291:657–661
Haydon PG (2001) GLIA: listening and talking to the synapse. Nat Rev Neurosci 2:185–193
Perea G, Araque A (2006) Synaptic information processing by astrocytes. J Physiol Paris 99:92–97
Ghashghaei HT, Lai C, Anton ES (2007) Neuronal migration in the adult brain: are we there yet? Nat Rev Neurosci 8:141–151
Mittal B, David S (1994) The role of an astrocyte surface molecule in neuronal migration in the developing rat cerebellum. Mol Cell Neurosci 5:78–86
Perea G, Araque A (2010) GLIA modulates synaptic transmission. Brain Res Rev 63:93–102
Shigetomi E, Bowser DN, Sofroniew MV, Khakh BS (2008) Two forms of astrocyte calcium excitability have distinct effects on NMDA receptor-mediated slow inward currents in pyramidal neurons. J Neurosci 28:6659–6663
Koehler RC, Roman RJ, Harder DR (2009) Astrocytes and the regulation of cerebral blood flow. Trends Neurosci 32:160–169
Gordon GR, Mulligan SJ, MacVicar BA (2007) Astrocyte control of the cerebrovasculature. Glia 55:1214–1221
Schummers J, Yu H, Sur M (2008) Tuned responses of astrocytes and their influence on hemodynamic signals in the visual cortex. Science 320:1638–1643
Araya R, Kudo M, Kawano M, Ishii K, Hashikawa T et al (2008) BMP signaling through BMPRIA in astrocytes is essential for proper cerebral angiogenesis and formation of the blood-brain-barrier. Mol Cell Neurosci 38:417–430
Rubin LL, Staddon JM (1999) The cell biology of the blood–brain barrier. Annu Rev Neurosci 22:11–28
Obara M, Szeliga M, Albrecht J (2008) Regulation of pH in the mammalian central nervous system under normal and pathological conditions: facts and hypotheses. Neurochem Int 52:905–919
Tom VJ, Doller CM, Malouf AT, Silver J (2004) Astrocyte-associated fibronectin is critical for axonal regeneration in adult white matter. J Neurosci 24:9282–9290
Bartus K, James ND, Bosch KD, Bradbury EJ (2012) Chondroitin sulphate proteoglycans: Key modulators of spinal cord and brain plasticity. Exp Neurol 235:5–17
Galtrey CM, Fawcett JW (2007) The role of chondroitin sulfate proteoglycans in regeneration and plasticity in the central nervous system. Brain Res Rev 54:1–18
Gris P, Tighe A, Levin D, Sharma R, Brown A (2007) Transcriptional regulation of scar gene expression in primary astrocytes. Glia 55:1145–1155
McKeon RJ, Hoke A, Silver J (1995) Injury-induced proteoglycans inhibit the potential for laminin-mediated axon growth on astrocytic scars. Exp Neurol 136:32–43
Silver J (1994) Inhibitory molecules in development and regeneration. J Neurol 242:S22–S24
Pekny M, Wilhelmsson U, Bogestal YR, Pekna M (2007) The role of astrocytes and complement system in neural plasticity. Int Rev Neurobiol 82:95–111
Pekny M, Nilsson M (2005) Astrocyte activation and reactive gliosis. Glia 50:427–434
Ridet JL, Malhotra SK, Privat A, Gage FH (1997) Reactive astrocytes: cellular and molecular cues to biological function. Trends Neurosci 20:570–577
Eddleston M, Mucke L (1993) Molecular profile of reactive astrocytes—implications for their role in neurologic disease. Neuroscience 54:15–36
Ajtai BM, Kálmán M (2001) Reactive glia support and guide axon growth in the rat thalamus during the first postnatal week. A sharply timed transition from permissive to non-permissive stage. Int J Dev Neurosci 19:589–597
Norenberg MD, Smith J, Marcillo A (2004) The pathology of human spinal cord injury: defining the problems. J Neurotrauma 21:429–440
Buss A, Pech K, Kakulas BA, Martin D, Schoenen J et al (2007) Matrix metalloproteinases and their inhibitors in human traumatic spinal cord injury. BMC Neurol 7:17
Hagg T, Oudega M (2006) Degenerative and spontaneous regenerative processes after spinal cord injury. J Neurotrauma 23:264–280
Bundesen LQ, Scheel TA, Bregman BS, Kromer LF (2003) Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. J Neurosci 23:7789–7800
Filbin MT (2006) Recapitulate development to promote axonal regeneration: good or bad approach? Philos Trans R Soc Lond B Biol Sci 361:1565–1574
Cafferty WB, McGee AW, Strittmatter SM (2008) Axonal growth therapeutics: regeneration or sprouting or plasticity? Trends Neurosci 31:215–220
Lu P, Tuszynski MH (2008) Growth factors and combinatorial therapies for CNS regeneration. Exp Neurol 209:313–320
Glabinski AR, Balasingam V, Tani M, Kunkel SL, Strieter RM et al (1996) Chemokine monocyte chemoattractant protein-1 is expressed by astrocytes after mechanical injury to the brain. J Immunol 156:4363–4368
Reilly JF, Kumari VG (1996) Alterations in fibroblast growth factor receptor expression following brain injury. Exp Neurol 140:139–150
Merrill JE, Benveniste EN (1996) Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci 19:331–338
Kahn MA, Huang CJ, Caruso A, Barresi V, Nazarian R et al (1997) Ciliary neurotrophic factor activates JAK/Stat signal transduction cascade and induces transcriptional expression of glial fibrillary acidic protein in glial cells. J Neurochem 68:1413–1423
Rabchevsky AG, Weinitz JM, Coulpier M, Fages C, Tinel M et al (1998) A role for transforming growth factor alpha as an inducer of astrogliosis. J Neurosci 18:10541–10552
Kordek R, Nerurkar VR, Liberski PP, Isaacson S, Yanagihara R et al (1996) Heightened expression of tumor necrosis factor alpha, interleukin 1 alpha, and glial fibrillary acidic protein in experimental Creutzfeldt-Jakob disease in mice. Proc Natl Acad Sci U S A 93:9754–9758
Lin HW, Basu A, Druckman C, Cicchese M, Krady JK et al (2006) Astrogliosis is delayed in type 1 interleukin-1 receptor-null mice following a penetrating brain injury. J Neuroinflammation 3:15
Vitellaro-Zuccarello L, Mazzetti S, Madaschi L, Bosisio P, Fontana E et al (2008) Chronic erythropoietin-mediated effects on the expression of astrocyte markers in a rat model of contusive spinal cord injury. Neuroscience 151:452–466
de Bilbao F, Arsenijevic D, Moll T, Garcia-Gabay I, Vallet P et al (2009) In vivo over-expression of interleukin-10 increases resistance to focal brain ischemia in mice. J Neurochem 110:12–22
Buffo A, Rolando C, Ceruti S (2010) Astrocytes in the damaged brain: molecular and cellular insights into their reactive response and healing potential. Biochem Pharmacol 79:77–89
Tada M, Diserens AC, Desbaillets I, de Tribolet N (1994) Analysis of cytokine receptor messenger RNA expression in human glioblastoma cells and normal astrocytes by reverse-transcription polymerase chain reaction. J Neurosurg 80:1063–1073
Aranguez I, Torres C, Rubio N (1995) The receptor for tumor necrosis factor on murine astrocytes: characterization, intracellular degradation, and regulation by cytokines and Theiler’s murine encephalomyelitis virus. Glia 13:185–194
Balasingam V, Yong VW (1996) Attenuation of astroglial reactivity by interleukin-10. J Neurosci 16:2945–2955
Aubert I, Ridet JL, Gage FH (1995) Regeneration in the adult mammalian CNS: guided by development. Curr Opin Neurobiol 5:625–635
Fitch MT, Doller C, Combs CK, Landreth GE, Silver J (1999) Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci 19:8182–8198
Munoz L, Ralay Ranaivo H, Roy SM, Hu W, Craft JM et al (2007) A novel p38 alpha MAPK inhibitor suppresses brain proinflammatory cytokine up-regulation and attenuates synaptic dysfunction and behavioral deficits in an Alzheimer’s disease mouse model. J Neuroinflammation 4:21
Fernandez AM, Fernandez S, Carrero P, Garcia-Garcia M, Torres-Aleman I (2007) Calcineurin in reactive astrocytes plays a key role in the interplay between proinflammatory and anti-inflammatory signals. J Neurosci 27:8745–8756
Chen Y, Miles DK, Hoang T, Shi J, Hurlock E et al (2008) The basic helix–loop–helix transcription factor olig2 is critical for reactive astrocyte proliferation after cortical injury. J Neurosci 28:10983–10989
Brambilla R, Bracchi-Ricard V, Hu WH, Frydel B, Bramwell A et al (2005) Inhibition of astroglial nuclear factor kappaB reduces inflammation and improves functional recovery after spinal cord injury. J Exp Med 202:145–156
Shafit-Zagardo B, Kume-Iwaki A, Goldman JE (1988) Astrocytes regulate GFAP mRNA levels by cyclic AMP and protein kinase C-dependent mechanisms. Glia 1:346–354
Schachtrup C, Ryu JK, Helmrick MJ, Vagena E, Galanakis DK et al (2010) Fibrinogen triggers astrocyte scar formation by promoting the availability of active TGF-beta after vascular damage. J Neurosci 30:5843–5854
Gadea A, Schinelli S, Gallo V (2008) Endothelin-1 regulates astrocyte proliferation and reactive gliosis via a JNK/c-Jun signaling pathway. J Neurosci 28:2394–2408
Okada S, Nakamura M, Katoh H, Miyao T, Shimazaki T et al (2006) Conditional ablation of Stat3 or Socs3 discloses a dual role for reactive astrocytes after spinal cord injury. Nat Med 12:829–834
John GR, Lee SC, Brosnan CF (2003) Cytokines: powerful regulators of glial cell activation. Neuroscientist 9:10–22
Li ZW, Tang RH, Zhang JP, Tang ZP, Qu WS, et al. (2012) Inhibiting epidermal growth factor receptor attenuates reactive astrogliosis and improves functional outcome after spinal cord injury in rats. Neurochem Int 58(7):812–819
Sahni V, Mukhopadhyay A, Tysseling V, Hebert A, Birch D et al (2010) BMPR1a and BMPR1b signaling exert opposing effects on gliosis after spinal cord injury. J Neurosci 30:1839–1855
Bareyre FM, Schwab ME (2003) Inflammation, degeneration and regeneration in the injured spinal cord: insights from DNA microarrays. Trends Neurosci 26:555–563
Goldshmit Y, Galea MP, Wise G, Bartlett PF, Turnley AM (2004) Axonal regeneration and lack of astrocytic gliosis in EphA4-deficient mice. J Neurosci 24:10064–10073
Davies JE, Tang X, Denning JW, Archibald SJ, Davies SJ (2004) Decorin suppresses neurocan, brevican, phosphacan and NG2 expression and promotes axon growth across adult rat spinal cord injuries. Eur J Neurosci 19:1226–1242
Logan A, Baird A, Berry M (1999) Decorin attenuates gliotic scar formation in the rat cerebral hemisphere. Exp Neurol 159:504–510
Logan A, Green J, Hunter A, Jackson R, Berry M (1999) Inhibition of glial scarring in the injured rat brain by a recombinant human monoclonal antibody to transforming growth factor-beta2. Eur J Neurosci 11:2367–2374
Bethea JR, Castro M, Keane RW, Lee TT, Dietrich WD et al (1998) Traumatic spinal cord injury induces nuclear factor-kappaB activation. J Neurosci 18:3251–3260
Saadoun S, Papadopoulos MC, Watanabe H, Yan D, Manley GT et al (2005) Involvement of aquaporin-4 in astroglial cell migration and glial scar formation. J Cell Sci 118:5691–5698
Nesic O, Lee J, Ye Z, Unabia GC, Rafati D et al (2006) Acute and chronic changes in aquaporin 4 expression after spinal cord injury. Neuroscience 143:779–792
Peters CM, Rogers SD, Pomonis JD, Egnaczyk GF, Keyser CP et al (2003) Endothelin receptor expression in the normal and injured spinal cord: potential involvement in injury-induced ischemia and gliosis. Exp Neurol 180:1–13
Egnaczyk GF, Pomonis JD, Schmidt JA, Rogers SD, Peters C et al (2003) Proteomic analysis of the reactive phenotype of astrocytes following endothelin-1 exposure. Proteomics 3:689–698
Lazarini F, Strosberg AD, Couraud PO, Cazaubon SM (1996) Coupling of ETB endothelin receptor to mitogen-activated protein kinase stimulation and DNA synthesis in primary cultures of rat astrocytes. J Neurochem 66:459–465
Rogers SD, Demaster E, Catton M, Ghilardi JR, Levin LA et al (1997) Expression of endothelin-B receptors by glia in vivo is increased after CNS injury in rats, rabbits, and humans. Exp Neurol 145:180–195
Rogers SD, Peters CM, Pomonis JD, Hagiwara H, Ghilardi JR et al (2003) Endothelin B receptors are expressed by astrocytes and regulate astrocyte hypertrophy in the normal and injured CNS. Glia 41:180–190
Koyama Y, Takemura M, Fujiki K, Ishikawa N, Shigenaga Y et al (1999) BQ788, an endothelin ET(B) receptor antagonist, attenuates stab wound injury-induced reactive astrocytes in rat brain. Glia 26:268–271
Teixeira A, Chaverot N, Strosberg AD, Cazaubon S (2000) Differential regulation of cyclin D1 and D3 expression in the control of astrocyte proliferation induced by endothelin-1. J Neurochem 74:1034–1040
Robel S, Mori T, Zoubaa S, Schlegel J, Sirko S et al (2009) Conditional deletion of beta1-integrin in astroglia causes partial reactive gliosis. Glia 57:1630–1647
Robel S, Bardehle S, Lepier A, Brakebusch C, Gotz M (2011) Genetic deletion of cdc42 reveals a crucial role for astrocyte recruitment to the injury site in vitro and in vivo. J Neurosci 31:12471–12482
Osmani N, Vitale N, Borg JP, Etienne-Manneville S (2006) Scrib controls Cdc42 localization and activity to promote cell polarization during astrocyte migration. Curr Biol 16:2395–2405
Etienne-Manneville S, Hall A (2001) Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell 106:489–498
Correa-Cerro LS, Mandell JW (2007) Molecular mechanisms of astrogliosis: new approaches with mouse genetics. J Neuropathol Exp Neurol 66:169–176
Mandell JW, VandenBerg SR (1999) ERK/MAP kinase is chronically activated in human reactive astrocytes. Neuroreport 10:3567–3572
Carbonell WS, Mandell JW (2003) Transient neuronal but persistent astroglial activation of ERK/MAP kinase after focal brain injury in mice. J Neurotrauma 20:327–336
Rolls A, Shechter R, Schwartz M (2009) The bright side of the glial scar in CNS repair. Nat Rev Neurosci 10:235–241
Mulligan SJ, MacVicar BA (2004) Calcium transients in astrocyte endfeet cause cerebrovascular constrictions. Nature 431:195–199
Rothstein JD, Dykes-Hoberg M, Pardo CA, Bristol LA, Jin L et al (1996) Knockout of glutamate transporters reveals a major role for astroglial transport in excitotoxicity and clearance of glutamate. Neuron 16:675–686
Vermeiren C, Najimi M, Vanhoutte N, Tilleux S, de Hemptinne I et al (2005) Acute up-regulation of glutamate uptake mediated by mGluR5a in reactive astrocytes. J Neurochem 94:405–416
Lindenau J, Noack H, Asayama K, Wolf G (1998) Enhanced cellular glutathione peroxidase immunoreactivity in activated astrocytes and in microglia during excitotoxin induced neurodegeneration. Glia 24:252–256
Menet V, Prieto M, Privat A, Gimenez y Ribotta M (2003) Axonal plasticity and functional recovery after spinal cord injury in mice deficient in both glial fibrillary acidic protein and vimentin genes. Proc Natl Acad Sci U S A 100:8999–9004
do Carmo Cunha J, de Freitas Azevedo Levy B, de Luca BA, de Andrade MS, Gomide VC et al (2007) Responses of reactive astrocytes containing S100beta protein and fibroblast growth factor-2 in the border and in the adjacent preserved tissue after a contusion injury of the spinal cord in rats: implications for wound repair and neuroregeneration. Wound Repair Regen 15:134–146
Stichel CC, Muller HW (1998) The CNS lesion scar: new vistas on an old regeneration barrier. Cell Tissue Res 294:1–9
Profyris C, Cheema SS, Zang D, Azari MF, Boyle K et al (2004) Degenerative and regenerative mechanisms governing spinal cord injury. Neurobiol Dis 15:415–436
Reier PJ, Houle JD (1988) The glial scar: its bearing on axonal elongation and transplantation approaches to CNS repair. Adv Neurol 47:87–138
Jones LL, Sajed D, Tuszynski MH (2003) Axonal regeneration through regions of chondroitin sulfate proteoglycan deposition after spinal cord injury: a balance of permissiveness and inhibition. J Neurosci 23:9276–9288
Garattini S, Mennini T, Samanin R (1987) From fenfluramine racemate to d-fenfluramine. Specificity and potency of the effects on the serotoninergic system and food intake. Ann N Y Acad Sci 499:156–166
McKeon RJ, Schreiber RC, Rudge JS, Silver J (1991) Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci 11:3398–3411
Mizuno H, Warita H, Aoki M, Itoyama Y (2008) Accumulation of chondroitin sulfate proteoglycans in the microenvironment of spinal motor neurons in amyotrophic lateral sclerosis transgenic rats. J Neurosci Res 86:2512–2523
Fitch MT, Silver J (1997) Activated macrophages and the blood-brain barrier: inflammation after CNS injury leads to increases in putative inhibitory molecules. Exp Neurol 148:587–603
Jones LL, Margolis RU, Tuszynski MH (2003) The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol 182:399–411
Stichel CC, Hermanns S, Luhmann HJ, Lausberg F, Niermann H et al (1999) Inhibition of collagen IV deposition promotes regeneration of injured CNS axons. Eur J Neurosci 11:632–646
Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Schut D, Fehlings MG (2010) Synergistic effects of transplanted adult neural stem/progenitor cells, chondroitinase, and growth factors promote functional repair and plasticity of the chronically injured spinal cord. J Neurosci 30:1657–1676
Barritt AW, Davies M, Marchand F, Hartley R, Grist J et al (2006) Chondroitinase ABC promotes sprouting of intact and injured spinal systems after spinal cord injury. J Neurosci 26:10856–10867
Massey JM, Hubscher CH, Wagoner MR, Decker JA, Amps J et al (2006) Chondroitinase ABC digestion of the perineuronal net promotes functional collateral sprouting in the cuneate nucleus after cervical spinal cord injury. J Neurosci 26:4406–4414
Fouad K, Schnell L, Bunge MB, Schwab ME, Liebscher T et al (2005) Combining Schwann cell bridges and olfactory-ensheathing glia grafts with chondroitinase promotes locomotor recovery after complete transection of the spinal cord. J Neurosci 25:1169–1178
Fry EJ, Chagnon MJ, Lopez-Vales R, Tremblay ML, David S (2010) Corticospinal tract regeneration after spinal cord injury in receptor protein tyrosine phosphatase sigma deficient mice. Glia 58:423–433
Yiu G, He Z (2006) Glial inhibition of CNS axon regeneration. Nat Rev Neurosci 7:617–627
Koprivica V, Cho KS, Park JB, Yiu G, Atwal J et al (2005) EGFR activation mediates inhibition of axon regeneration by myelin and chondroitin sulfate proteoglycans. Science 310:106–110
Erschbamer M, Pernold K, Olson L (2007) Inhibiting epidermal growth factor receptor improves structural, locomotor, sensory, and bladder recovery from experimental spinal cord injury. J Neurosci 27:6428–6435
Monnier PP, Sierra A, Schwab JM, Henke-Fahle S, Mueller BK (2003) The Rho/ROCK pathway mediates neurite growth-inhibitory activity associated with the chondroitin sulfate proteoglycans of the CNS glial scar. Mol Cell Neurosci 22:319–330
Jain A, McKeon RJ, Brady-Kalnay SM, Bellamkonda RV (2011) Sustained delivery of activated Rho GTPases and BDNF promotes axon growth in CSPG-rich regions following spinal cord injury. PLoS One 6:e16135
Jain A, Brady-Kalnay SM, Bellamkonda RV (2004) Modulation of Rho GTPase activity alleviates chondroitin sulfate proteoglycan-dependent inhibition of neurite extension. J Neurosci Res 77:299–307
Sivasankaran R, Pei J, Wang KC, Zhang YP, Shields CB et al (2004) PKC mediates inhibitory effects of myelin and chondroitin sulfate proteoglycans on axonal regeneration. Nat Neurosci 7:261–268
Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE (2002) Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci 22:10368–10376
Goldshmit Y, Spanevello MD, Tajouri S, Li L, Rogers F et al (2011) EphA4 blockers promote axonal regeneration and functional recovery following spinal cord injury in mice. PLoS One 6:e24636
Goldshmit Y, McLenachan S, Turnley A (2006) Roles of Eph receptors and ephrins in the normal and damaged adult CNS. Brain Res Rev 52:327–345
Miranda JD, White LA, Marcillo AE, Willson CA, Jagid J et al (1999) Induction of Eph B3 after spinal cord injury. Exp Neurol 156:218–222
Puschmann TB, Turnley AM (2010) Eph receptor tyrosine kinases regulate astrocyte cytoskeletal rearrangement and focal adhesion formation. J Neurochem 113:881–894
Song H, Stevens CF, Gage FH (2002) Astroglia induce neurogenesis from adult neural stem cells. Nature 417:39–44
Lim DA, Alvarez-Buylla A (1999) Interaction between astrocytes and adult subventricular zone precursors stimulates neurogenesis. Proc Natl Acad Sci U S A 96:7526–7531
Barkho BZ, Song H, Aimone JB, Smrt RD, Kuwabara T et al (2006) Identification of astrocyte-expressed factors that modulate neural stem/progenitor cell differentiation. Stem Cell Dev 15:407–421
Ueki T, Tanaka M, Yamashita K, Mikawa S, Qiu Z et al (2003) A novel secretory factor, neurogenesin-1, provides neurogenic environmental cues for neural stem cells in the adult hippocampus. J Neurosci 23:11732–11740
Karimi-Abdolrezaee S, Eftekharpour E, Wang J, Morshead CM, Fehlings MG (2006) Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J Neurosci 26:3377–3389
Barnabe-Heider F, Frisen J (2008) Stem cells for spinal cord repair. Cell Stem Cell 3:16–24
Barnabe-Heider F, Goritz C, Sabelstrom H, Takebayashi H, Pfrieger FW et al (2010) Origin of new glial cells in intact and injured adult spinal cord. Cell Stem Cell 7:470–482
Meletis K, Barnabe-Heider F, Carlen M, Evergren E, Tomilin N et al (2008) Spinal cord injury reveals multilineage differentiation of ependymal cells. PLoS Biol 6:e182
Horky LL, Galimi F, Gage FH, Horner PJ (2006) Fate of endogenous stem/progenitor cells following spinal cord injury. J Comp Neurol 498:525–538
Siebert JR, Osterhout DJ (2011) The inhibitory effects of chondroitin sulfate proteoglycans on oligodendrocytes. J Neurochem 119:176–188
Siebert JR, Stelzner DJ, Osterhout DJ (2011) Chondroitinase treatment following spinal contusion injury increases migration of oligodendrocyte progenitor cells. Exp Neurol 231:19–29
Wang Y, Cheng X, He Q, Zheng Y, Kim DH et al (2011) Astrocytes from the contused spinal cord inhibit oligodendrocyte differentiation of adult oligodendrocyte precursor cells by increasing the expression of bone morphogenetic proteins. J Neurosci 31:6053–6058
Pekny M (2001) Astrocytic intermediate filaments: lessons from GFAP and vimentin knock-out mice. Progr Brain Res 132:23–30
Yick LW, Cheung PT, So KF, Wu W (2003) Axonal regeneration of Clarke’s neurons beyond the spinal cord injury scar after treatment with chondroitinase ABC. Exp Neurol 182:160–168
Gama CI, Tully SE, Sotogaku N, Clark PM, Rawat M et al (2006) Sulfation patterns of glycosaminoglycans encode molecular recognition and activity. Nat Chem Biol 2:467–473
Caggiano AO, Zimber MP, Ganguly A, Blight AR, Gruskin EA (2005) Chondroitinase ABCI improves locomotion and bladder function following contusion injury of the rat spinal cord. J Neurotrauma 22:226–239
Garcia-Alias G, Lin R, Akrimi SF, Story D, Bradbury EJ et al (2008) Therapeutic time window for the application of chondroitinase ABC after spinal cord injury. Exp Neurol 210:331–338
Grimpe B, Silver J (2004) A novel DNA enzyme reduces glycosaminoglycan chains in the glial scar and allows microtransplanted dorsal root ganglia axons to regenerate beyond lesions in the spinal cord. J Neurosci 24:1393–1397
Rolls A, Shechter R, London A, Segev Y, Jacob-Hirsch J et al (2008) Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med 5:e171
Kwok JC, Afshari F, Garcia-Alias G, Fawcett JW (2008) Proteoglycans in the central nervous system: plasticity, regeneration and their stimulation with chondroitinase ABC. Restor Neurol Neurosci 26:131–145
Garcia-Alias G, Barkhuysen S, Buckle M, Fawcett JW (2009) Chondroitinase ABC treatment opens a window of opportunity for task-specific rehabilitation. Nat Neurosci 12:1145–1151
Tyor WR, Avgeropoulos N, Ohlandt G, Hogan EL (2002) Treatment of spinal cord impact injury in the rat with transforming growth factor-beta. J Neurol Sci 200:33–41
Ihn H (2008) Autocrine TGF-beta signaling in the pathogenesis of systemic sclerosis. J Dermatol Sci 49:103–113
Mittaud P, Labourdette G, Zingg H, Guenot-Di Scala D (2002) Neurons modulate oxytocin receptor expression in rat cultured astrocytes: involvement of TGF-beta and membrane components. Glia 37:169–177
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Karimi-Abdolrezaee, S., Billakanti, R. Reactive Astrogliosis after Spinal Cord Injury—Beneficial and Detrimental Effects. Mol Neurobiol 46, 251–264 (2012). https://doi.org/10.1007/s12035-012-8287-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12035-012-8287-4