Abstract
Deficits in neuronal function are a hallmark of spinal cord injury (SCI) and therapeutic efforts are often focused on central nervous system (CNS) axon regeneration. However, secondary injury responses by astrocytes, microglia, pericytes, endothelial cells, Schwann cells, fibroblasts, meningeal cells, and other glia not only potentiate SCI damage but also facilitate endogenous repair. Due to their profound impact on the progression of SCI, glial cells and modification of the glial scar are focuses of SCI therapeutic research. Within and around the glial scar, cells deposit extracellular matrix (ECM) proteins that affect axon growth such as chondroitin sulfate proteoglycans (CSPGs), laminin, collagen, and fibronectin. This dense deposition of material, i.e., the fibrotic scar, is another barrier to endogenous repair and is a target of SCI therapies. Infiltrating neutrophils and monocytes are recruited to the injury site through glial chemokine and cytokine release and subsequent upregulation of chemotactic cellular adhesion molecules and selectins on endothelial cells. These peripheral immune cells, along with endogenous microglia, drive a robust inflammatory response to injury with heterogeneous reparative and pathological properties and are targeted for therapeutic modification. Here, we review the role of glial and inflammatory cells after SCI and the therapeutic strategies that aim to replace, dampen, or alter their activity to modulate SCI scarring and inflammation and improve injury outcomes.
Similar content being viewed by others
Introduction: Glial Effectors of Spinal Cord Injury Scarring and Inflammation
Neuronal dysfunction underlies the disabilities associated with spinal cord injury (SCI). At the time of injury synaptic connections are lost, demyelination and axon damage disrupts signal propagation, and neurons undergo mechanically induced cell death. The primary injury also activates a secondary cascade of vascular, inflammatory, and biochemical events that further disrupt neuronal function. These primary and secondary injury events activate glia, including astrocytes, fibroblasts, pericytes, Schwann cells, and microglia. The dialog between activated glia and injured neurons underlies endogenous pathological and reparative processes in the injured central nervous system (CNS).
In the absence of injury, glia support signal transmission and neuronal function. Oligodendrocytes wrap axons with myelin sheaths, insulating the axon to increase action potential conduction velocity and decrease signal decrement. Astrocytes interface with the vasculature and sequester and transport neurotransmitters, ions, and nutrients to neurons to optimize signaling. Pericytes ensheath endothelial cells of the CNS capillaries and can adjust capillary diameter, control vascular coupling, and control neurovascular function [1,2,3]. Microglia patrol the CNS as resident immune cells and sample the CNS environment phagocytosing potential pathogens while secreting growth and supportive factors.
Following SCI, glia secrete toxins and cytokines in response to the mechanical damage. Tissue initially spared from mechanical trauma is susceptible to secondary damage from these glial by-products [4]. The diverse assemblage of glial cells necessary to maintain healthy CNS function becomes a complicated array of cells now activated with pathological and reparative properties. The mechanical trauma and downstream signaling cascades further drive injury progression by facilitating infiltration of nonresident cells. Immune cells extravasate into the injury site and persist chronically within the injured spinal cord [5,6,7]. Fibroblasts either infiltrate from the periphery or differentiate from other resident cells and deposit inhibitory extracellular matrix (ECM) components within the injured spinal cord [2, 8]. Schwann cells migrate through dorsal root entry zones into the lesion epicenter and contribute ECM proteins and growth factors to the lesion milieu [9,10,11,12]. Collectively, SCI triggers diverse glial activation and cellular recruitment with complex downstream effects on neuronal function. Here, we will review the cellular effectors contributing to scarring and inflammation following SCI with a specific focus on glial-targeted therapies.
Glial and Fibrotic Scarring After Spinal Cord Injury
SCI activates resident astrocytes and pericytes, as well as recruits infiltrating fibroblasts and Schwann cells from periphery, leading to the development of lasting glial (cellular) and fibrotic (acellular) scars in the injured spinal cord (Table 1). Regarding the various cells of the glial scar, astrocytes surround the lesion site and take up residence in the lesion penumbra [42]. Pericytes and nonpericyte perivascular cells infiltrate into the lesion core where they are closely associated with ECM components such as fibronectin, laminin, and collagen, as well as, traditional fibroblast markers [2, 8, 26, 43]. The exact origin and contribution of these particular cells typically associated with connective tissue (i.e., pericytes, meningeal cells) is an active area of debate [2, 8, 44]; we will collectively refer to these cells as fibroblasts. Schwann cells from nerve peripheral roots infiltrate into the lesion epicenter where they also express fibroblast markers and closely associate with laminin, fibronectin, and collagen deposits [9,10,11,12].
The astrocytic and fibroblast/Schwann cell components of the glial scar are strictly separated to the penumbra and lesion core, respectively (Fig. 1). Indeed, many studies use astrocytic boundaries to demarcate regions of frank tissue pathology from more intact penumbral tissues [45]. This interface is sometimes referred to as the “glia limitans.” The strict sequestration of cell types is in stark contrast to regenerating species where both ECM components and glial cells cross the lesion site and precede neural regeneration [46,47,48]. Formation of the glia limitans may be species-specific or driven by cellular interactions, as the phenomenon has been replicated in vitro by cocultures of mammalian astrocytes and fibroblasts/Schwann cells that maintain spatial separation and inhibit neurite growth [11, 21, 42].
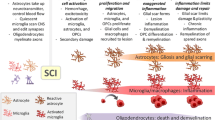
Schematic of resident and infiltrating glial cells and associated therapies following traumatic spinal cord injury. Resident microglia and astrocytes are activated following injury and form a glial scar surrounding and sequestering the damaged tissue (top). Fibroblasts and inflammatory cells infiltrate into the damaged tissue and deposit extracellular matrix proteins forming the fibrous scar (middle). Activated cells exacerbate damage, leading to an expanded secondary injury. Therapeutic approaches (bold) and example agents (hyphenated) targeting glial activation, scar formation, and inflammation after spinal cord injury (bottom). This therapeutic list is not comprehensive, and references and abbreviations are in the main body of the manuscript
After injury, proliferating astrocytes thicken cellular processes and surround the lesion with a meshwork of overlapping outgrowths (Fig. 1). Astrocyte activation and subsequent glial scar boundaries are enhanced by the addition of transforming growth factor-beta (TGF-β) [21, 49, 50]. TGF-β increases microglia/macrophage and astrocyte activation and fibronectin and laminin deposition [49]. Signal transducer and activator of transcription 3 (STAT3) is also important for establishing the glial scar border that secludes infiltrating cells to the lesion epicenter [51, 52]. Previous schools of thought simply classified the glial scar as a maladaptation opposing neurite regrowth. More recently, evidence indicates that the glial scar is important for neurotrophin production, debris clearance, blood brain barrier repair, and toxic species sequestration to the injury site [13, 53]. The positive role of the glial scar in SCI responses is reflected by the necessity of a glial bridge for neural regeneration in nonmammalian models [46, 48].
The fibrotic scar, i.e., the acellular components of the scar consisting of deposited ECM materials, influences the cellular distribution of the glial scar. ECM molecules can increase the rigidity of the environment, create a physical barrier, and provide nonspecific topographical cues, all of which may affect cellular migration (Fig. 1) [54,55,56]. Additionally, ECM components signal through cell surface receptors to influence cellular activity. For example, tenascin and fibronectin increase matrix metalloproteases (MMPs) in various cell types [57,58,59] and MMPs influence outcomes of SCI including the infiltration of cells into the injury core [60,61,62,63,64,65,66]. Despite the presence of tenascin, fibronectin, and MMPs at the glia limitans, the demarcation remains intact chronically. Overall, investigations into the glia limitans provide interesting pathophysiological descriptions but therapeutic strategies that interfere with the establishment of the scar demarcations or that drive the injury responses toward establishing a glial bridge are limited.
The fibrotic scar is also a critical regulator of axonal regeneration and growth after SCI (Table 1). Cells within the lesion core mediate ECM dynamics through production of ECM components and proteolytic enzymes, especially MMPs [26, 67, 68]. MMPs degrade ECM molecules allowing receptor mediated assembly into dense matrices [26]. Several of the ECM components, such as CSPGs and fibronectin, inhibit neurite regrowth in vitro; others, such as laminin, promote greater neurite outgrowth [13, 53, 55, 69,70,71,72]. Similarly, removal of inhibitory ECM components, such as CSPGs, improves neurite growth in vivo [23, 46,47,48, 73], whereas removal of other ECM proteins, such as collagens, fails to promote regeneration or recovery [28]. The orientation and stiffness of ECM scaffolds may also act as a physical cue for neurite growth leading to strategies with aligning ECM components to promote directional axon growth [55, 56, 70, 74, 75].
Using transgenic models, researchers have gained insight into therapeutic targets that reduce the inhibitory effects of scarring on SCI repair. Targeted suppression of astrocyte signaling pathways reduces inhibitory scar formation and facilitates axon growth and SCI recovery [76]. Specifically, transgenic approaches have identified astrocyte inhibition of TGF-β/Smad, TLR, JAK/STAT3, and JNK/c-Jun signaling cascades, among others, as potential SCI therapies [76]. However, depending upon the timing post-injury, astrocyte inhibition also interferes with ECM deposition of growth-supportive substrates and neurotrophins (e.g., laminin, fibronectin, growth factors) thereby reducing endogenous repair processes [77]. Indeed, transgenic models demonstrate that astrocytes play an important role in limiting the spread of secondary injury events early after injury [14]. Although the results of these transgenic models reveal a complex role for scarring after injury, there are ongoing research efforts to target different components of glial and fibrotic scars to improve SCI recovery.
The above cellular components of the glial scar and ECM deposition of the fibrotic scar are primarily derived from observations made in rodent SCI models. By comparison, data is limited regarding SCI scar formation in humans. As in rodents, there is clear cellular demarcation of the glial scar with astrocytes around the lesion border and fibroblasts, Schwann cells, and meningeal cells sequestered within the lesion [9, 10, 78,79,80,81]. This inverse relationship between astrocytes and other glial cells within the lesion is similar between species. However, in humans, Schwann cells are the predominant cells type composing the glial scar within the lesion instead of fibroblasts as in rodents [9, 10, 79, 80]. There are also conflicting reports suggesting that the prominence of the astrocytic glial scar varies between species with less astrocytosis in humans [10, 80].
The fibrotic scar, i.e., the acellular components of the scar consisting of deposited ECM materials, is sometimes referred to as the mesenchymal or fibroblastic scar in humans [80]. The composition of the fibrotic scar in humans consists of abundant collagen, laminin, and fibronectin deposits within the lesion core and reduced deposition in penumbral areas of astrocyte activation [10, 80]. Although the distribution and prominence of CSPGs are comparable between species, there is evidence that the cellular specificity and spatiotemporal distribution of specific CSPGs may vary between humans and rodents. For example, in humans, versican and neurocan are found almost exclusively within the lesion and are likely produced by Schwann cells rather than by fibroblasts, glial precursors, meningeal cells, or astrocytes (in the lesion penumbra) as observed in rats [9, 24, 80, 82]. Collectively, there is strong evidence that Schwann cells disproportionately contribute to the glial and fibrotic scar in human versus rodent SCI.
Spinal Cord Injury Therapies Targeting the Glial and Fibrotic Scar
Therapies targeting astrocyte activation and the glial scar focus primarily on three approaches (Fig. 1). The first approach is to break down inhibitory extracellular matrix molecules produced by astrocytes with CSPGs being the primary target. The second is to transplant astrocyte stem cells, or glial-restricted precursor cells, into the injured spinal cord to suppress scar formation, facilitate a permissive environment through the release of growth factors, deposit supportive ECM substrates, and increase the effectiveness of stem cell therapies. The third is to manipulate the extracellular matrix through transplantation of biomaterials that provide substrates for axon growth and effectively bypass or alter the inhibitory glial ECM deposition that occurs after SCI.
As mentioned above, the glial and fibrotic scars form a physical and chemical barrier to axon growth. The dense deposition of extracellular matrix presents a physical obstruction for growing axons. In addition, CSGPs and other inhibitory extracellular matrix molecules bind receptors that signal axonal growth inhibition [83]. Transgenic manipulation of SOX9 and N-acetylgalactosaminyl transferase demonstrate the efficacy of reducing CSPGs on SCI neuroprotection and axon regeneration [84, 85]. Therapeutically, enzymatic digestion of CSPGs with anti-XT-1 DNA enzyme or chondroitinase ABC (chABC) facilitates axon regeneration and functional recovery [23, 86, 87]. There is promising converging preclinical evidence of increased axon growth after SCI with the chABC treatment from multiple independent researchers and in combination with other therapeutic strategies (reviewed by [88]). Chondroitinase ABC may also provide anti-inflammatory mediated neuroprotection by reducing pro-inflammatory CSPG stimuli [89,90,91]. Across a number of different rodent SCI models, chABC treatment improves functional recovery [88]. As mentioned above, the presence and distribution of CSPGs after SCI are similar between rodents and humans, and therefore, optimizations in delivery and safety may lead to successful translation of chABC treatment to humans [88].
Interestingly, glial crosstalk may contribute to the therapeutic effects of chABC treatment. Chondroitinase ABC has immunomodulatory effects when delivered after SCI as shown by the increased predominance of reparative macrophages with treatment [90, 92]. Specifically, the immunomodulatory effects of chABC treatment depend in part on the release of IL-10, an anti-inflammatory cytokine that increases reparative (also called “M2”—see inflammation below) macrophage activation [92]. Blocking IL-10 with neutralizing antibodies reduces chABC-mediated reparative macrophage activation in vivo [92]. In addition, IL-10 may play an essential role in the dialog between infiltrating macrophages and astrocyte-mediated ECM depositions after SCI [67, 93]. The immunomodulatory changes associated with chABC treatment highlight the complex interactions among glia cells related to SCI therapies [94, 95].
The cellular sources and extracellular components of the glial and fibrotic scars are still being identified [2, 8, 96]. Other therapeutic approaches targeted to reduce fibrotic and glial scar formation include suppression of TGF-beta, an upstream regulator of fibroblast proliferation; iron chelation to decrease fibrotic ECM formation; and epothilone B and D treatment to limit pericyte and fibroblast proliferation and migration [29, 32, 97, 98]. Of these, the iron chelator, deferoxamine, and epothilone D have viable safety profiles in humans; however, further work is likely required for both agents to understand their mechanisms of action in SCI. The therapeutic effects of epothilone D may be due to microtubule stabilization in axons. Indeed, a recent evaluation of epothilone D mediated SCI recovery was unable to clearly define the anatomical correlates of treatment [99]. Similarly, the therapeutic effects of deferoxamine after contusion SCI are likely multifaceted and include changes not only in scars but also in inflammation and apoptosis [100].
A second therapeutic approach targeting the fibrotic and glial scars is the transplantation of immature astrocytes into the injured spinal cord. Immature astrocytes, either derived from developmentally immature CNS tissue or immature with regard to lineage progression, support axon growth after injury [101]. A comprehensive review of astrocyte-based stem cell therapies was recently written by Angelo Lepore and colleagues [101] and another review in this special edition focuses on cellular transplantation strategies. In our experience, transplanted glial-restricted precursor cells differentiate into both oligodendrocytes and astrocytes and decrease glial limitans formation and proteoglycan expression concurrent with increased axon regeneration and sprouting [102]. Interestingly, we detected no significant changes in overt inflammatory responses with transplantation, but we did not examine the phenotype of infiltrating macrophages [102]. Schwann cell transplantation is also associated with glial and fibrotic scar changes and is discussed in detail in the companion transplantation chapter of this special edition.
A third therapeutic approach involves manipulation of the ECM through the transplantation of biomaterials. A clinical illustration of this approach comes from a small, ongoing phase I trial in China (ClinicalTrials.gov: NCT02352077) [103, 104]. The goal of the clinical trial is to create a permissive extracellular environment through transplantation of a linearly oriented scaffold that serves both as a delivery tool for bone marrow mononuclear cells (BMMCs) [103] or mesenchymal stem cells (MSC) [104] and to orient axon regeneration across the injured spinal cord. The combinatorial approach has the potential to overcome endogenous molecular and physical ECM barriers after SCI. The transplanted cells release growth factors to counter molecular inhibition and the construction of scaffolds with a growth-supportive matrix (i.e., collagen) facilitates axon growth across physical glial barriers. Preliminary results of the phase I trial in eight patients receiving MSCs with complete chronic SCI report partial recovery of motor function in three patients and changes in autonomic function in six patients with no adverse effects 1 year after surgery [104]. Similar results were reported from 5 patients that received BMMCs, there were no significant adverse effects for 12 months postoperatively and 2 individuals had partial recovery of sexual arousal and somatosensory evoked potentials [103]. Researchers have tested difference biomaterials and cellular sources in preclinical models with promising results, further demonstrating the therapeutic potential of targeting the glial and fibrotic scar after SCI [105].
Acute Inflammation Following Spinal Cord Injury
Spinal cord injury creates cellular debris and releases intracellular proteins that act as potent inflammatory stimuli. These injury-exposed debris signals, also called damage-associated molecular patterns (DAMPs), are normally concealed from immune surveillance within the intact CNS [106]. After injury, DAMPs engage pattern recognition receptors (PRRs) on inflammatory cells used to detect foreign microbes that invade the body [107]. The results are rapid DAMP- and PRR-mediated activation of resident inflammatory cells including astrocytes and microglia [35]. Reactive astrocytes and microglia release a wide variety of oxidative stress regulators, cytokines, chemokines, growth factors and other inflammatory mediators [108]. Microglia also alter cellular morphology and protein expression profiles after SCI. Under normal conditions, microglia have long, thin processes that extend out from the central cell body to sample the extracellular environment. Following injury, microglia retract their processes and assume a more amoeboid morphology better equipped for phagocytosis and debris clearance. These activated cells closely resemble circulating macrophages in their morphology, protein expression profile, and function [109].
Along with the morphological changes comes the release of chemokines and cytokines which serve to recruit peripheral neutrophils and macrophages into the injured spinal cord [110]. Chemokines drive increased expression of selectins and cell adhesion proteins on nearby endothelial cells. Integrin-mediated adhesion of circulating immune cells facilitates extravasation of monocytes and neutrophils into the spinal cord [111]. The first wave of infiltrating immune cells are neutrophils, which, in rodents and humans, peak within the spinal cord around 1 day post-injury (dpi) [2, 5, 6, 38, 39, 80, 112, 113]. Neutrophils perform bactericidal functions as the first line of defense against invaders; however, following SCI, by-products of neutrophil-mediated phagocytosis of opsonized particles and degranulation of proteases including reactive oxygen species are primarily considered cytotoxic [11, 26, 43, 114]. Due to the hallmark presence of myeloperoxidase and their ability to mount a potentially destructive oxidative burst, neutrophils are purported contributors to SCI pathology in experimental models. However, conflicting studies report varying degrees of neutrophil-mediated oxidative damage following rodent SCI [46,47,48, 114,115,116]. Neutrophils persist chronically at low levels in the injured mouse spinal cord but decrease within a week of injury in both rodents and humans [5, 6, 38, 80, 113, 117] coincident with increased monocyte-derived macrophages infiltration into the spinal cord [35].
Infiltrating macrophages contribute proteolytic enzymes, reactive oxygen species, and inflammatory cytokines to the injury microenvironment but also perform necessary functions of debris clearance, cellular remodeling, and production of pro-regenerative factors [109, 110, 118, 119]. The dual beneficial and reparative functions of macrophages make understanding their role in the injury response difficult. Endogenous microglia-derived and recruited monocyte-derived macrophages are also difficult to distinguish in the injured spinal cord. As discussed above, macrophages are very similar to microglia in morphology, protein expression, and function. Indeed, disentangling the two cell types required flow cytometry or genetic methods until very recent identification of protein markers distinct to the microglia [36, 120]. Nonetheless, favorable and unfavorable outcomes are associated with the inhibition of inflammatory cell recruitment following SCI [39, 40].
Researchers now discuss the beneficial versus pathological roles of macrophages in SCI through subcategorization of macrophages into a variety of activation states [110]. Categorization of these activation states in SCI has been revisited several times in recent years beginning with the identification of endogenously activated pathological M1, or “classically activated,” and reparative M2, or “alternatively activated,” macrophages in the injured spinal cord [37]. Alternative activation states are sometimes subdivided into M2a, M2b, and M2c with more recent trends favoring a indistinct view of macrophage phenotype in SCI in which the same cell can exhibit a diversity of both M1 and M2 markers [109, 110, 121].
Regardless of terminology, researchers recognize that macrophages not only can increase axon regeneration and neuronal function but can also exacerbate tissue destruction [119, 122]. Unfortunately, pro-inflammatory M1 macrophages predominate after injury in rodents [110] and there is evidence of a sustained M1-like monocyte activation after human SCI [123]. Due to the diverse role of macrophages in both injury and repair, therapeutically, clinicians and scientists are developing immunomodulatory approaches for potentiating reparative, M2, microglia and macrophage activation within the injured spinal cord. Past experimental and clinical attempts involved transplantation of prestimulated exogenous microglia or macrophages [124,125,126]. With the identification of endogenously activated reparative microglia and macrophages after SCI [37], more recent immunomodulatory therapeutic approaches are focused on polarizing endogenous cells toward a reparative phenotype.
Neuroprotective Spinal Cord Injury Therapies Targeting Inflammation
To date, only one pharmacological therapy, methylprednisolone, has completed phase III clinical trials with demonstrated efficacy [127]. Interestingly, the therapeutic effect of this corticosteroid is due in part to its anti-inflammatory properties including decreased macrophage activation. Although methylprednisolone remains the only clinically approved treatment for SCI, its use has declined in recent decades. The decline is due to perceived risks associated with corticosteroid treatment (i.e., gastrointestinal bleeding and wound infection) and a potentially limited therapeutic value and treatment window (8 h) [127]. Despite the current debates regarding its use [128], methylprednisolone provides clinical evidence that limiting inflammation, specifically microglia/macrophage activation, is neuroprotective in SCI.
More recently, therapeutics targeting macrophages and microglia primarily focused on two approaches. The first involves targeting infiltrating immune cells through pharmacological macrophage depletion or antibody-based approaches to interrupt endothelial–monocyte interactions with the ultimate goal of reducing macrophage activation in the injury site. The second focuses on immunomodulation and promotion of reparative, M2, macrophages using pharmacological and transplantation therapies.
Antibodies that disrupt monocyte-endothelial cell interactions result in decreased tissue loss and increased functional recovery in rodent models of SCI. Specifically, extensive work by Dekaban, Weaver, and colleagues provides comprehensive evidence that the mechanism of action for antibodies targeted to CD11d/CD18 or α4β1 integrins involve reduced microglia and macrophage accumulation within the injured spinal cord [111, 129,130,131,132,133,134,135,136,137,138,139,140]. Although neutrophils may also be affected by treatment [141], these data implicate monocyte-derived macrophages, and potentially CD11d expressing microglia, as mediators of secondary injury after SCI. Further, these data also demonstrate that upregulation of selectins and cell adhesion molecules on endothelial cells after injury may potentiate destructive neuroinflammation.
CD11d-mediated depletion is effective in both rats and mice after SCI [142] regardless of injury type (i.e., compression vs contusion) and in various models of traumatic brain injury [131, 132, 143]. In contrast, SCI treatment with clodronate liposomes, a drug that induces selective deletion of phagocytic monocyte-derived macrophages [144], reduces indices of secondary injury but with inconsistent functional recovery [15, 40, 145, 146]. Similarly, depletion of circulating monocytes using silica dust or chloroquine and colchicine leads to improved function after SCI but these effects have not been replicated in 25 years and new evidence suggests mechanisms of action independent of macrophage inhibition [147,148,149].
The inconsistency in functional recovery between depletion-type approaches and anti-integrin antibodies are likely due to the heterogeneity of monocyte subsets activated by SCI. It is possible that CD11d selectively targets entry of pathological macrophages, whereas more general depletion approaches limit both reparative and pathological populations. Indeed, there is emerging evidence that specific monocyte subpopulations reduce inflammation and scar formation after SCI [93]. Consistent with this concept of heterogeneity, selective depletion of monocytes expressing the macrophage receptor with collagenous structure (MARCO), a receptor associated with pro-inflammatory macrophage activation [150], leads to improved functional recovery and increased axon sprouting after SCI [151]. Further, specific monocyte subsets expressing the fractalkine receptor, CX3CR1, mediate axon retraction and potentiate anatomical and functional impairments after SCI [152, 153]. Natalizumab, an antibody against α4β1, is effective in multiple sclerosis and similar therapies have been evaluated after myocardial infarction and stroke in humans [154,155,156]. To the best of our knowledge, the effectiveness of anti-integrin antibody therapies for human SCI remains untested. Regarding approaches involving infiltrating myeloid cells in SCI, targeted depletion that accounts for potential functional monocyte heterogeneity may be of the most significant clinical impact [157].
The most direct approach for increasing reparative macrophages and microglia after SCI involves transplanting prestimulated cells into the injured spinal cord. Specifically, transplantation of cultured microglia or macrophages prestimulated by anti-inflammatory cytokines, peripheral nerve segments, or cocultured with skin, to induce reparative phenotypes, increases axon growth and functional recovery after rat SCI [125, 126, 158, 159]. The observations that macrophages may facilitate repair in the injured spinal cord formed the scientific rationale for the ProCord clinical trials sponsored by ProNeuron Biotechnologies. The design and experimental evidence, as well as issues with patient recruitment and demographics for ProCord, have been discussed in detail previously [95, 124, 160, 161]. Briefly, autologous macrophages were isolated from SCI individuals and cocultured in autologous skin biopsies. After activation, these purportedly reparative macrophages were then transplanted into the injured spinal cord. The results of a phase 1 trial on 8 patients indicated that the cells were well tolerated and three patients experienced functional improvements after transplantation [161]. However, a larger scale, phase II trial with 43 participants failed to detect a significant effect of macrophage transplantation and reported a trend toward increased functional recovery in the control group [162]. Although ultimately unsuccessful, the ProNeuron trial demonstrated the therapeutic feasibility of transplantation trials in SCI [163].
The effects of current cellular therapies for SCI (reviewed in the accompanying special issue article) may be due in part to transplantation-mediated macrophage polarization toward reparative phenotypes. For example, MSCs, neuronal stems cells, olfactory ensheathing cells, and Schwann cells may release anti-inflammatory cytokines as transplantation of these cells into the injured spinal cord is associated with activation of endogenous M2-like macrophages and microglia [95, 164, 165]. Transplant-associated changes in macrophage and microglia activation states provide indirect evidence that modulating inflammatory cell phenotypes may be therapeutic.
More direct evidence comes from efficacy associated with the application of cytokines, specifically IL-4, that drive M2 macrophage activation in vitro [166]. Either systemic or intraspinal administration of IL-4 after SCI increases production of the anti-inflammatory cytokine, IL-10, coincident with increases in markers associated with M2 macrophage activation [167, 168]. IL-4 administration also reduces iNOS, a purported mediator of M1 neurotoxicity, regardless of administration route [167, 168]. In addition, IL-4 treatment facilitates neuroprotection as indicated by increased tissue sparing and functional recovery [167, 168]. Other anti-inflammatory cytokines and growth factors including intraspinal delivery of IL-37, systemic delivery of granulocyte colony-stimulating factor, and cell-mediated delivery of IL-13 (a hallmark cytokine that induces M2 activation) facilitate similar effects [169,170,171]. Although not all anti-inflammatory cytokine therapies are effective in SCI [172], data from these preclinical rodent studies indicate that driving increased M2 macrophage activation is a promising therapeutic approach for treating SCI.
The counter approach, blocking pro-inflammatory cytokines that induce M1 activation, is also beneficial in SCI. Specifically, application of MR16-1, a monoclonal antibody against the prototypical pro-inflammatory cytokine IL-6, decreases iNOS- and CD16/32-positive M1 macrophages and increases arginase-1- and CD206-positive M2 macrophages in the injured spinal cord [173]. These immunomodulatory shifts are coincident with increased tissue sparing and functional recovery [173]. The therapeutic effects of IL-6 inhibition may not be due entirely to immunomodulatory changes in macrophage/microglia, as IL-6 inhibition also alters astrocyte activation [174, 175]. Nonetheless, blocking other pro-inflammatory mediators such as TNFα and macrophage migration inhibitory factor (MIF) after SCI facilitates wound resolution and improves recovery [176, 177]. Collectively, the results of the converging approaches of increasing M2 activation through the delivery of anti-inflammatory cytokines and blocking M1 activation through the delivery of blocking antibodies or inhibitors support applying immunomodulatory therapies to treat SCI.
As an alternative to direct manipulation of pro- or anti-inflammatory cytokines, researchers are also investigating immunomodulation using clinically tolerated pharmacological approaches. The list of drugs and natural compounds with immunomodulatory properties in SCI has grown in recent years and is too extensive to discuss here thoroughly. Some pharmaceutical agents with desirable clinical safety profiles and demonstrated immunomodulatory properties in SCI include the antibiotics minocycline and azithromycin [45, 150, 178] and natural compounds such as docosahexaenoic/omega-3 fatty acids and flavonoids [179, 180]. These immunomodulatory agents facilitate functional recovery and reduce indices of secondary injury. For a more comprehensive review of immunomodulatory therapies in spinal cord injury, see the following reviews: [121, 164, 181].
Conclusion
Scarring and inflammatory responses to SCI include a complex diversity of cells and cellular activities that vary based on injury type, timing, and spatial distribution [182, 183]. Glial and inflammatory cells affect the injury progression with profound impacts on overall neuronal function and SCI outcomes. The importance of SCI glial and fibrotic scarring, as well as inflammation, has naturally led researchers and scientists to target these responses for therapeutic intervention. Although researchers have found variable amounts of success, unfortunately, few therapies make it to clinical trials and there are no mainstream therapies for SCI.
In our opinion, the general aspects of SCI scarring and inflammation in humans are recapitulated in rodent models of injury. Due to the importance of these complex and intertwined SCI responses, animal models are pivotal for building a holistic understanding of SCI progression and repair. However, subtle and potentially therapeutically relevant differences in cellular composition and timing exist among species. Therapies that can account for these differences, as well as the intertwined role that glia and hematogenous myeloid cells play in scarring responses to SCI, may have the greatest potential for translational success. Further, researchers may need to look to transgenic and novel regeneration models to develop a regenerative roadmap that successfully traverses the complex SCI scarring and inflammatory landscape. As we continue to broaden our understanding of the diverse components of the SCI microenvironment, it is likely that we will find endogenous keys to unlock SCI regeneration and repair.
References
Bell RD, Winkler EA, Sagare AP, et al. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron. 68(3), 409–427 (2010).
Göritz C, Dias DO, Tomilin N, Barbacid M, Shupliakov O, Frisén J. A pericyte origin of spinal cord scar tissue. Science. 333(6039), 238–242 (2011).
Peppiatt CM, Howarth C, Mobbs P, Attwell D. Bidirectional control of CNS capillary diameter by pericytes. Nature. 443(7112), 700–704 (2006).
Oyinbo CA. Secondary injury mechanisms in traumatic spinal cord injury: a nugget of this multiply cascade. Acta Neurobiol Exp (Wars). 71(2), 281–299 (2011).
Beck KD, Nguyen HX, Galvan MD, Salazar DL, Woodruff TM, Anderson AJ. Quantitative analysis of cellular inflammation after traumatic spinal cord injury: evidence for a multiphasic inflammatory response in the acute to chronic environment. Brain. 133(Pt 2), 433–447 (2010).
Fleming JC, Norenberg MD, Ramsay DA, et al. The cellular inflammatory response in human spinal cords after injury. Brain. 129(Pt 12), 3249–3269 (2006).
Sroga JM, Jones TB, Kigerl KA, McGaughy VM, Popovich PG. Rats and mice exhibit distinct inflammatory reactions after spinal cord injury. J Comp Neurol. 462(2), 223–240 (2003).
Soderblom C, Luo X, Blumenthal E, et al. Perivascular fibroblasts form the fibrotic scar after contusive spinal cord injury. Journal of Neuroscience. 33(34), 13882–13887 (2013).
Bruce JH, Norenberg MD, Kraydieh S, Puckett W, Marcillo A, Dietrich D. Schwannosis: role of gliosis and proteoglycan in human spinal cord injury. J Neurotrauma. 17(9), 781–788 (2000).
Buss A, Pech K, Kakulas BA, et al. Growth-modulating molecules are associated with invading Schwann cells and not astrocytes in human traumatic spinal cord injury. Brain. 130(Pt 4), 940–953 (2007).
Zhang S-X, Huang F, Gates M, Holmberg EG. Role of endogenous Schwann cells in tissue repair after spinal cord injury. Neural Regen Res. 8(2), 177–185 (2013).
Beattie MS, Bresnahan JC, Komon J, et al. Endogenous repair after spinal cord contusion injuries in the rat. Exp Neurol. 148(2), 453–463 (1997).
Anderson MA, Burda JE, Ren Y, et al. Astrocyte scar formation aids central nervous system axon regeneration. Nature. 532(7598), 195–200 (2016).
Burda JE, Sofroniew MV. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron. 81(2), 229–248 (2014).
Zhu Y, Soderblom C, Krishnan V, Ashbaugh J, Bethea JR, Lee JK. Hematogenous macrophage depletion reduces the fibrotic scar and increases axonal growth after spinal cord injury. Neurobiol Dis. 74C, 114–125 (2014).
Zamanian JL, Xu L, Foo LC, et al. Genomic analysis of reactive astrogliosis. Journal of Neuroscience. 32(18), 6391–6410 (2012).
David S, Aguayo AJ. Axonal elongation into peripheral nervous system “bridges” after central nervous system injury in adult rats. Science. 214(4523), 931–933 (1981).
Abnet K, Fawcett JW, Dunnett SB. Interactions between meningeal cells and astrocytes in vivo and in vitro. Brain Res. Dev. Brain Res. 59(2), 187–196 (1991).
Bundesen LQ, Scheel TA, Bregman BS, Kromer LF. Ephrin-B2 and EphB2 regulation of astrocyte-meningeal fibroblast interactions in response to spinal cord lesions in adult rats. Journal of Neuroscience. 23(21), 7789–7800 (2003).
Shearer MC, Fawcett JW. The astrocyte/meningeal cell interface—a barrier to successful nerve regeneration? Cell Tissue Res. 305(2), 267–273 (2001).
Kimura-Kuroda J, Teng X, Komuta Y, et al. An in vitro model of the inhibition of axon growth in the lesion scar formed after central nervous system injury. Mol. Cell. Neurosci. 43(2), 177–187 (2010).
Kawano H, Kimura-Kuroda J, Komuta Y, et al. Role of the lesion scar in the response to damage and repair of the central nervous system. Cell Tissue Res. 349(1), 169–180 (2012).
Bradbury EJ, Moon LDF, Popat RJ, et al. Chondroitinase ABC promotes functional recovery after spinal cord injury. Nature. 416(6881), 636–640 (2002).
Tang X, Davies JE, Davies SJA. Changes in distribution, cell associations, and protein expression levels of NG2, neurocan, phosphacan, brevican, versican V2, and tenascin-C during acute to chronic maturation of spinal cord scar tissue. J Neurosci Res. 71(3), 427–444 (2003).
McKeon RJ, Jurynec MJ, Buck CR. The chondroitin sulfate proteoglycans neurocan and phosphacan are expressed by reactive astrocytes in the chronic CNS glial scar. Journal of Neuroscience. 19(24), 10778–10788 (1999).
Zhu Y, Soderblom C, Trojanowsky M, Lee D-H, Lee JK. Fibronectin matrix assembly after spinal cord injury. J Neurotrauma. 32(15), 1158–1167 (2015).
Schreiber J, Schachner M, Schumacher U, Lorke DE. Extracellular matrix alterations, accelerated leukocyte infiltration and enhanced axonal sprouting after spinal cord hemisection in tenascin-C-deficient mice. Acta Histochem. 115(8), 865–878 (2013).
Weidner N, Grill RJ, Tuszynski MH. Elimination of basal lamina and the collagen “scar” after spinal cord injury fails to augment corticospinal tract regeneration. Exp Neurol. 160(1), 40–50 (1999).
Klapka N, Hermanns S, Straten G, et al. Suppression of fibrous scarring in spinal cord injury of rat promotes long-distance regeneration of corticospinal tract axons, rescue of primary motoneurons in somatosensory cortex and significant functional recovery. Eur J Neurosci. 22(12), 3047–3058 (2005).
Loy DN, Crawford CH, Darnall JB, Burke DA, Onifer SM, Whittemore SR. Temporal progression of angiogenesis and basal lamina deposition after contusive spinal cord injury in the adult rat. J Comp Neurol. 445(4), 308–324 (2002).
Klapka N, Müller HW. Collagen matrix in spinal cord injury. J Neurotrauma. 23(3–4), 422–435 (2006).
Ruschel J, Hellal F, Flynn KC, et al. Axonal regeneration. Systemic administration of epothilone B promotes axon regeneration after spinal cord injury. Science. 348(6232), 347–352 (2015).
McKeon RJ, Schreiber RC, Rudge JS, Silver J. Reduction of neurite outgrowth in a model of glial scarring following CNS injury is correlated with the expression of inhibitory molecules on reactive astrocytes. J Neurosci. 11(11), 3398–3411 (1991).
Stichel CC, Niermann H, D'Urso D, Lausberg F, Hermanns S, Müller HW. Basal membrane-depleted scar in lesioned CNS: characteristics and relationships with regenerating axons. Neuroscience. 93(1), 321–333 (1999).
Donnelly DJ, Popovich PG. Inflammation and its role in neuroprotection, axonal regeneration and functional recovery after spinal cord injury. Exp Neurol. 209(2), 378–388 (2008).
Mawhinney LA, Thawer SG, Lu W-Y, et al. Differential detection and distribution of microglial and hematogenous macrophage populations in the injured spinal cord of lys-EGFP-ki transgenic mice. J Neuropathol Exp Neurol. 71(3), 180–197 (2012).
Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. Journal of Neuroscience. 29(43), 13435–13444 (2009).
Carlson SL, Parrish ME, Springer JE, Doty K, Dossett L. Acute inflammatory response in spinal cord following impact injury. Exp Neurol. 151(1), 77–88 (1998).
Taoka Y, Okajima K, Uchiba M, et al. Role of neutrophils in spinal cord injury in the rat. Neuroscience. 79(4), 1177–1182 (1997).
Popovich PG, Guan Z, Wei P, Huitinga I, van Rooijen N, Stokes BT. Depletion of hematogenous macrophages promotes partial hindlimb recovery and neuroanatomical repair after experimental spinal cord injury. Exp Neurol. 158(2), 351–365 (1999).
Wang G, Zhang J, Hu X, et al. Microglia/macrophage polarization dynamics in white matter after traumatic brain injury. J Cereb Blood Flow Metab. 33(12), 1864–1874 (2013).
Fitch MT, Doller C, Combs CK, Landreth GE, Silver J. Cellular and molecular mechanisms of glial scarring and progressive cavitation: in vivo and in vitro analysis of inflammation-induced secondary injury after CNS trauma. J Neurosci. 19(19), 8182–8198 (1999).
Soderblom C, Lee D-H, Dawood A, et al. 3D imaging of axons in transparent spinal cords from rodents and nonhuman primates. eNeuro. 2(2) (2015).
Decimo I, Bifari F, Rodriguez FJ, et al. Nestin- and doublecortin-positive cells reside in adult spinal cord meninges and participate in injury-induced parenchymal reaction. STEM CELLS. 29(12), 2062–2076 (2011).
Zhang B, Bailey WM, Kopper TJ, Orr MB, Feola DJ, Gensel JC. Azithromycin drives alternative macrophage activation and improves recovery and tissue sparing in contusion spinal cord injury. J Neuroinflammation. 12, 218 (2015).
Bloom O. Non-mammalian model systems for studying neuro-immune interactions after spinal cord injury. Exp Neurol. 258, 130–140 (2014).
Goldshmit Y, Sztal TE, Jusuf PR, Hall TE, Nguyen-Chi M, Currie PD. Fgf-dependent glial cell bridges facilitate spinal cord regeneration in zebrafish. Journal of Neuroscience. 32(22), 7477–7492 (2012).
Zukor KA, Kent DT, Odelberg SJ. Meningeal cells and glia establish a permissive environment for axon regeneration after spinal cord injury in newts. Neural Dev. 6, 1 (2011).
Logan A, Berry M, Gonzalez AM, Frautschy SA, Sporn MB, Baird A. Effects of transforming growth factor beta 1 on scar production in the injured central nervous system of the rat. Eur J Neurosci. 6(3), 355–363 (1994).
East E, Golding JP, Phillips JB. A versatile 3D culture model facilitates monitoring of astrocytes undergoing reactive gliosis. J Tissue Eng Regen Med. 3(8), 634–646 (2009).
Renault-Mihara F, Mukaino M, Shinozaki M, et al. Regulation of RhoA by STAT3 coordinates glial scar formation. J Cell Biol. 216(8), 2533–2550 (2017).
Wanner IB, Anderson MA, Song B, et al. Glial scar borders are formed by newly proliferated, elongated astrocytes that interact to corral inflammatory and fibrotic cells via STAT3-dependent mechanisms after spinal cord injury. Journal of Neuroscience. 33(31), 12870–12886 (2013).
Faulkner JR, Herrmann JE, Woo MJ, Tansey KE, Doan NB, Sofroniew MV. Reactive astrocytes protect tissue and preserve function after spinal cord injury. J Neurosci. 24(9), 2143–2155 (2004).
Bott K, Upton Z, Schrobback K, et al. The effect of matrix characteristics on fibroblast proliferation in 3D gels. Biomaterials. 31(32), 8454–8464 (2010).
Harris GM, Madigan NN, Lancaster KZ, et al. Nerve guidance by a decellularized fibroblast extracellular matrix. Matrix Biol. 60-61, 176–189 (2017).
Franze K, Janmey PA, Guck J. Mechanics in neuronal development and repair. Annu Rev Biomed Eng. 15, 227–251 (2013).
Tremble P, Chiquet-Ehrismann R, Werb Z. The extracellular matrix ligands fibronectin and tenascin collaborate in regulating collagenase gene expression in fibroblasts. Mol. Biol. Cell. 5(4), 439–453 (1994).
Trebaul A, Chan EK, Midwood KS. Regulation of fibroblast migration by tenascin-C. Biochem. Soc. Trans. 35(Pt 4), 695–697 (2007).
Kalembeyi I, Inada H, Nishiura R, Imanaka-Yoshida K, Sakakura T, Yoshida T. Tenascin-C upregulates matrix metalloproteinase-9 in breast cancer cells: direct and synergistic effects with transforming growth factor beta1. Int. J. Cancer. 105(1), 53–60 (2003).
Ogier C, Bernard A, Chollet A-M, et al. Matrix metalloproteinase-2 (MMP-2) regulates astrocyte motility in connection with the actin cytoskeleton and integrins. Glia. 54(4), 272–284 (2006).
Goussev S, Hsu J-YC, Lin Y, et al. Differential temporal expression of matrix metalloproteinases after spinal cord injury: relationship to revascularization and wound healing. J. Neurosurg. 99(2 Suppl), 188–197 (2003).
Tezel G, Hernandez MR, Wax MB. In vitro evaluation of reactive astrocyte migration, a component of tissue remodeling in glaucomatous optic nerve head. Glia. 34(3), 178–189 (2001).
Takenaga K, Kozlova EN. Role of intracellular S100A4 for migration of rat astrocytes. Glia. 53(3), 313–321 (2006).
Yu F, Kamada H, Niizuma K, Endo H, Chan PH. Induction of mmp-9 expression and endothelial injury by oxidative stress after spinal cord injury. J Neurotrauma. 25(3), 184–195 (2008).
Hsu J-YC, McKeon R, Goussev S, et al. Matrix metalloproteinase-2 facilitates wound healing events that promote functional recovery after spinal cord injury. Journal of Neuroscience. 26(39), 9841–9850 (2006).
Zhang H, Trivedi A, Lee J-U, et al. Matrix metalloproteinase-9 and stromal cell-derived factor-1 act synergistically to support migration of blood-borne monocytes into the injured spinal cord. Journal of Neuroscience. 31(44), 15894–15903 (2011).
Shechter R, Raposo C, London A, Sagi I, Schwartz M. The glial scar-monocyte interplay: a pivotal resolution phase in spinal cord repair. PLoS ONE. 6(12), e27969 (2011).
Rolls A, Shechter R, London A, et al. Two faces of chondroitin sulfate proteoglycan in spinal cord repair: a role in microglia/macrophage activation. PLoS Med. 5(8), e171 (2008).
Tasdemir-Yilmaz OE, Freeman MR. Astrocytes engage unique molecular programs to engulf pruned neuronal debris from distinct subsets of neurons. Genes Dev. 28(1), 20–33 (2014).
Clark P, Britland S, Connolly P. Growth cone guidance and neuron morphology on micropatterned laminin surfaces. Journal of Cell Science. 105 ( Pt 1), 203–212 (1993).
Chung W-S, Clarke LE, Wang GX, et al. Astrocytes mediate synapse elimination through MEGF10 and MERTK pathways. Nature. 504(7480), 394–400 (2013).
Bush TG, Puvanachandra N, Horner CH, et al. Leukocyte infiltration, neuronal degeneration, and neurite outgrowth after ablation of scar-forming, reactive astrocytes in adult transgenic mice. Neuron. 23(2), 297–308 (1999).
Alilain WJ, Horn KP, Hu H, Dick TE, Silver J. Functional regeneration of respiratory pathways after spinal cord injury. Nature. 475(7355), 196–200 (2011).
Manwaring ME, Walsh JF, Tresco PA. Contact guidance induced organization of extracellular matrix. Biomaterials. 25(17), 3631–3638 (2004).
Gonzalez-Perez F, Udina E, Navarro X. Extracellular matrix components in peripheral nerve regeneration. Int. Rev. Neurobiol. 108, 257–275 (2013).
Shen D, Wang X, Gu X. Scar-modulating treatments for central nervous system injury. Neurosci Bull. 30(6), 967–984 (2014).
O’Shea TM, Burda JE, Sofroniew MV. Cell biology of spinal cord injury and repair. J Clin Invest. 127(9), 3259–3270 (2017).
Buss A, Brook GA, Kakulas B, et al. Gradual loss of myelin and formation of an astrocytic scar during Wallerian degeneration in the human spinal cord. Brain. 127(Pt 1), 34–44 (2004).
Buss A, Pech K, Kakulas BA, et al. NG2 and phosphacan are present in the astroglial scar after human traumatic spinal cord injury. BMC Neurol. 9, 32 (2009).
Norenberg MD, Smith J, Marcillo A. The pathology of human spinal cord injury: defining the problems. J Neurotrauma. 21(4), 429–440 (2004).
Guest JD, Hiester ED, Bunge RP. Demyelination and Schwann cell responses adjacent to injury epicenter cavities following chronic human spinal cord injury. Exp Neurol. 192(2), 384–393 (2005).
Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 182(2), 399–411 (2003).
Shen Y, Tenney AP, Busch SA, et al. PTPsigma is a receptor for chondroitin sulfate proteoglycan, an inhibitor of neural regeneration. Science. 326(5952), 592–596 (2009).
McKillop WM, Dragan M, Schedl A, Brown A. Conditional Sox9 ablation reduces chondroitin sulfate proteoglycan levels and improves motor function following spinal cord injury. Glia. 61(2), 164–177 (2013).
Takeuchi K, Yoshioka N, Higa Onaga S, et al. Chondroitin sulphate N-acetylgalactosaminyl-transferase-1 inhibits recovery from neural injury. Nat Commun. 4, 2740 (2013).
Oudega M, Chao OY, Avison DL, et al. Systemic administration of a deoxyribozyme to xylosyltransferase-1 mRNA promotes recovery after a spinal cord contusion injury. Exp Neurol. 237(1), 170–179 (2012).
Grimpe B, Silver J. A novel DNA enzyme reduces glycosaminoglycan chains in the glial scar and allows microtransplanted dorsal root ganglia axons to regenerate beyond lesions in the spinal cord. Journal of Neuroscience. 24(6), 1393–1397 (2004).
Bradbury EJ, Carter LM. Manipulating the glial scar: chondroitinase ABC as a therapy for spinal cord injury. Brain Res. Bull. 84(4–5), 306–316 (2011).
Carter LM, Starkey ML, Akrimi SF, Davies M, Mcmahon SB, Bradbury EJ. The yellow fluorescent protein (YFP-H) mouse reveals neuroprotection as a novel mechanism underlying chondroitinase ABC-mediated repair after spinal cord injury. Journal of Neuroscience. 28(52), 14107–14120 (2008).
Bartus K, James ND, Didangelos A, et al. Large-scale chondroitin sulfate proteoglycan digestion with chondroitinase gene therapy leads to reduced pathology and modulates macrophage phenotype following spinal cord contusion injury. Journal of Neuroscience. 34(14), 4822–4836 (2014).
Xu X, Bass B, McKillop WM, et al. Sox9 knockout mice have improved recovery following stroke. Exp Neurol. 303, 59–71 (2018).
Didangelos A, Iberl M, Vinsland E, Bartus K, Bradbury EJ. Regulation of IL-10 by chondroitinase ABC promotes a distinct Immune response following spinal cord injury. Journal of Neuroscience. 34(49), 16424–16432 (2014).
Shechter R, London A, Varol C, et al. Infiltrating blood-derived macrophages are vital cells playing an anti-inflammatory role in recovery from spinal cord injury in mice. PLoS Med. 6(7), e1000113 (2009).
Gensel JC, Kigerl KA, Mandrekar-Colucci SS, Gaudet AD, Popovich PG. Achieving CNS axon regeneration by manipulating convergent neuro-immune signaling. Cell Tissue Res. 349(1), 201–213 (2012).
Gensel JC, Donnelly DJ, Popovich PG. Spinal cord injury therapies in humans: an overview of current clinical trials and their potential effects on intrinsic CNS macrophages. Expert Opin. Ther. Targets. 15(4), 505–518 (2011).
Hesp ZC, Yoseph RY, Suzuki R, Wilson C, Nishiyama A, McTigue DM. Proliferating NG2 cell-dependent angiogenesis and scar formation alter axon growth and functional recovery after spinal cord injury in mice. Journal of Neuroscience. (2017).
Zhao W, Chai Y, Hou Y, et al. Mechanisms responsible for the inhibitory effects of epothilone B on scar formation after spinal cord injury. Neural Regen Res. 12(3), 478–485 (2017).
Ruschel J, Bradke F. Systemic administration of epothilone D improves functional recovery of walking after rat spinal cord contusion injury. Exp Neurol. (2017).
Sandner B, Puttagunta R, Motsch M, et al. Systemic epothilone D improves hindlimb function after spinal cord contusion injury in rats. Exp Neurol. (2018).
Hao J, Li B, Duan H-Q, et al. Mechanisms underlying the promotion of functional recovery by deferoxamine after spinal cord injury in rats. Neural Regen Res. 12(6), 959–968 (2017).
Falnikar A, Li K, Lepore AC. Therapeutically targeting astrocytes with stem and progenitor cell transplantation following traumatic spinal cord injury. Brain Res. 1619, 91–103 (2015).
Hill CE, Proschel C, Noble M, et al. Acute transplantation of glial-restricted precursor cells into spinal cord contusion injuries: survival, differentiation, and effects on lesion environment and axonal regeneration. Exp Neurol. 190(2), 289–310 (2004).
Xiao Z, Tang F, Tang J, et al. One-year clinical study of NeuroRegen scaffold implantation following scar resection in complete chronic spinal cord injury patients. Sci China Life Sci. 59(7), 647–655 (2016).
Zhao Y, Tang F, Xiao Z, et al. Clinical study of NeuroRegen scaffold combined with human mesenchymal stem cells for the repair of chronic complete spinal cord injury. Cell Transplant. 26(5), 891–900 (2017).
Haggerty AE, Marlow MM, Oudega M. Extracellular matrix components as therapeutics for spinal cord injury. Neurosci Lett. 652, 50–55 (2017).
Kigerl KA, Popovich PG. Toll-like receptors in spinal cord injury. Curr. Top. Microbiol. Immunol. 336, 121–136 (2009).
Kigerl KA, de Rivero Vaccari JP, Dietrich WD, Popovich PG, Keane RW. Pattern recognition receptors and central nervous system repair. Exp Neurol. 258, 5–16 (2014).
Sofroniew MV. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 32(12), 638–647 (2009).
David S, Kroner A. Repertoire of microglial and macrophage responses after spinal cord injury. Nat Rev Neurosci. 12(7), 388–399 (2011).
Gensel JC, Zhang B. Macrophage activation and its role in repair and pathology after spinal cord injury. Brain Res. 1619, 1–11 (2015).
Mabon PJ, Weaver LC, Dekaban GA. Inhibition of monocyte/macrophage migration to a spinal cord injury site by an antibody to the integrin alphaD: a potential new anti-inflammatory treatment. Exp Neurol. 166(1), 52–64 (2000).
Yang L, Blumbergs PC, Jones NR, Manavis J, Sarvestani GT, Ghabriel MN. Early expression and cellular localization of proinflammatory cytokines interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in human traumatic spinal cord injury. Spine. 29(9), 966–971 (2004).
Kigerl KA, McGaughy VM, Popovich PG. Comparative analysis of lesion development and intraspinal inflammation in four strains of mice following spinal contusion injury. J Comp Neurol. 494(4), 578–594 (2006).
Gensel JC, Popovich PG. Controversies on the role of inflammation in the injured spinal cord. In: Traumatic brain and spinal cord injury: challenges and developments in research. Morganti-Kossmann MC, Maas AI, Raghupathi R (Eds.). Cambrige Press, New York, 272–279.
de Castro R, Hughes MG, Xu GY, et al. Evidence that infiltrating neutrophils do not release reactive oxygen species in the site of spinal cord injury. Exp Neurol. 190(2), 414–424 (2004).
Kubota K, Saiwai H, Kumamaru H, et al. Myeloperoxidase exacerbates secondary injury by generating highly reactive oxygen species and mediating neutrophil recruitment in experimental spinal cord injury. Spine. 37(16), 1363–1369 (2012).
Prüss H, Kopp MA, Brommer B, et al. Non-resolving aspects of acute inflammation after spinal cord injury (SCI): indices and resolution plateau. Brain Pathology (Zurich, Switzerland). 21(6), 652–660 (2011).
Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu. Rev. Immunol. 27, 451–483 (2009).
Gensel JC, Nakamura S, Guan Z, Van Rooijen N, Ankeny DP, Popovich PG. Macrophages promote axon regeneration with concurrent neurotoxicity. Journal of Neuroscience. 29(12), 3956–3968 (2009).
Greenhalgh AD, Passos Dos Santos R, Zarruk JG, Salmon CK, Kroner A, David S. Arginase-1 is expressed exclusively by infiltrating myeloid cells in CNS injury and disease. Brain Behav Immun. (2016).
Ren Y, Young W. Managing inflammation after spinal cord injury through manipulation of macrophage function. Neural Plasticity. 2013, 945034 (2013).
Benowitz LI, Popovich PG. Inflammation and axon regeneration. Curr. Opin. Neurol. 24(6), 577–583 (2011).
Huang W, Vodovotz Y, Kusturiss MB, et al. Identification of distinct monocyte phenotypes and correlation with circulating cytokine profiles in acute response to spinal cord injury: a pilot study. PM&R. 6(4), 332–341 (2014).
Kigerl K, Popovich P. Drug evaluation: ProCord—a potential cell-based therapy for spinal cord injury. IDrugs. 9(5), 354–360 (2006).
Rapalino O, Lazarov-Spiegler O, Agranov E, et al. Implantation of stimulated homologous macrophages results in partial recovery of paraplegic rats. Nat Med. 4(7), 814–821 (1998).
Rabchevsky AG, Streit WJ. Grafting of cultured microglial cells into the lesioned spinal cord of adult rats enhances neurite outgrowth. J Neurosci Res. 47(1), 34–48 (1997).
Bracken MB, Shepard MJ, Collins WF, et al. A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury. Results of the Second National Acute Spinal Cord Injury Study. N. Engl. J. Med. 322(20), 1405–1411 (1990).
Bowers CA, Kundu B, Hawryluk GWJ. Methylprednisolone for acute spinal cord injury: an increasingly philosophical debate. Neural Regen Res. 11(6), 882–885 (2016).
Geremia NM, Bao F, Rosenzweig TE, et al. CD11d antibody treatment improves recovery in spinal cord-injured mice. J Neurotrauma. 29(3), 539–550 (2012).
Bao F, Brown A, Dekaban GA, Omana V, Weaver LC. CD11d integrin blockade reduces the systemic inflammatory response syndrome after spinal cord injury. Exp Neurol. 231(2), 272–283 (2011).
Shultz SR, Bao F, Weaver LC, Cain DP, Brown A. Treatment with an anti-CD11d integrin antibody reduces neuroinflammation and improves outcome in a rat model of repeated concussion. J Neuroinflammation. 10, 26 (2013).
Bao F, Shultz SR, Hepburn JD, et al. A CD11d monoclonal antibody treatment reduces tissue injury and improves neurological outcome after fluid percussion brain injury in rats. J Neurotrauma. 29(14), 2375–2392 (2012).
Saville LR, Pospisil CH, Mawhinney LA, et al. A monoclonal antibody to CD11d reduces the inflammatory infiltrate into the injured spinal cord: a potential neuroprotective treatment. J Neuroimmunol. 156(1–2), 42–57 (2004).
Bao F, Dekaban GA, Weaver LC. Anti-CD11d antibody treatment reduces free radical formation and cell death in the injured spinal cord of rats. J Neurochem. 94(5), 1361–1373 (2005).
Oatway MA, Chen Y, Bruce JC, Dekaban GA, Weaver LC. Anti-CD11d integrin antibody treatment restores normal serotonergic projections to the dorsal, intermediate, and ventral horns of the injured spinal cord. Journal of Neuroscience. 25(3), 637–647 (2005).
Gris D, Marsh DR, Oatway MA, et al. Transient blockade of the CD11d/CD18 integrin reduces secondary damage after spinal cord injury, improving sensory, autonomic, and motor function. J Neurosci. 24(16), 4043–4051 (2004).
Ditor DS, Bao F, Chen Y, Dekaban GA, Weaver LC. A therapeutic time window for anti-CD 11d monoclonal antibody treatment yielding reduced secondary tissue damage and enhanced behavioral recovery following severe spinal cord injury. J Neurosurg Spine. 5(4), 343–352 (2006).
Bao F, Chen Y, Dekaban GA, Weaver LC. Early anti-inflammatory treatment reduces lipid peroxidation and protein nitration after spinal cord injury in rats. J Neurochem. 88(6), 1335–1344 (2004).
Bao F, Omana V, Brown A, Weaver LC. The systemic inflammatory response after spinal cord injury in the rat is decreased by α4β1 integrin blockade. J Neurotrauma. 29(8), 1626–1637 (2012).
Fleming JC, Bao F, Chen Y, Hamilton EF, Relton JK, Weaver LC. Alpha4beta1 integrin blockade after spinal cord injury decreases damage and improves neurological function. Exp Neurol. 214(2), 147–159 (2008).
Plemel JR, Wee Yong V, Stirling DP. Immune modulatory therapies for spinal cord injury—past, present and future. Exp Neurol. 258, 91–104 (2014).
Kwon BK, Okon E, Hillyer J, et al. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J Neurotrauma. 28(8), 1545–1588 (2011).
Utagawa A, Bramlett HM, Daniels L, et al. Transient blockage of the CD11d/CD18 integrin reduces contusion volume and macrophage infiltration after traumatic brain injury in rats. Brain Res. 1207, 155–163 (2008).
Van Rooijen N, Hendrikx E. Liposomes for specific depletion of macrophages from organs and tissues. Methods Mol Biol. 605, 189–203 (2010).
Iannotti CA, Clark M, Horn KP, Van Rooijen N, Silver J, Steinmetz MP. A combination immunomodulatory treatment promotes neuroprotection and locomotor recovery after contusion SCI. Exp Neurol. 230(1), 3–15 (2011).
Horn KP, Busch SA, Hawthorne AL, Van Rooijen N, Silver J. Another barrier to regeneration in the CNS: activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. Journal of Neuroscience. 28(38), 9330–9341 (2008).
Wu F, Wei X, Wu Y, et al. Chloroquine promotes the recovery of acute spinal cord injury by inhibiting autophagy-associated inflammation and endoplasmic reticulum stress. J Neurotrauma. (2018).
Giulian D, Chen J, Ingeman JE, George JK, Noponen M. The role of mononuclear phagocytes in wound healing after traumatic injury to adult mammalian brain. J Neurosci. 9(12), 4416–4429 (1989).
Blight AR. Effects of silica on the outcome from experimental spinal cord injury: implication of macrophages in secondary tissue damage. Neuroscience. 60(1), 263–273 (1994).
Gensel JC, Kopper TJ, Zhang B, Orr MB, Bailey WM. Predictive screening of M1 and M2 macrophages reveals the immunomodulatory effectiveness of post spinal cord injury azithromycin treatment. Sci. Rep. 7, 40144 (2017).
Jeong SJ, Cooper JG, Ifergan I, et al. Intravenous immune-modifying nanoparticles as a therapy for spinal cord injury in mice. Neurobiol Dis. 108, 73–82 (2017).
Evans TA, Barkauskas DS, Myers JT, et al. High-resolution intravital imaging reveals that blood-derived macrophages but not resident microglia facilitate secondary axonal dieback in traumatic spinal cord injury. Exp Neurol. 254C, 109–120 (2014).
Donnelly DJ, Longbrake EE, Shawler TM, et al. Deficient CX3CR1 signaling promotes recovery after mouse spinal cord injury by limiting the recruitment and activation of Ly6Clo/iNOS+ macrophages. Journal of Neuroscience. 31(27), 9910–9922 (2011).
Saxena A, Russo I, Frangogiannis NG. Inflammation as a therapeutic target in myocardial infarction: learning from past failures to meet future challenges. Transl Res. 167(1), 152–166 (2016).
Polman CH, O'Connor PW, Havrdova E, et al. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N. Engl. J. Med. 354(9), 899–910 (2006).
Elkins J, Veltkamp R, Montaner J, et al. Safety and efficacy of natalizumab in patients with acute ischaemic stroke (ACTION): a randomised, placebo-controlled, double-blind phase 2 trial. Lancet Neurol. 16(3), 217–226 (2017).
Hawthorne AL, Popovich PG. Emerging concepts in myeloid cell biology after spinal cord injury. Neurotherapeutics. 8(2), 252–261 (2011).
Bomstein Y, Marder JB, Vitner K, et al. Features of skin-coincubated macrophages that promote recovery from spinal cord injury. J Neuroimmunol. 142(1–2), 10–16 (2003).
Ma S-F, Chen Y-J, Zhang J-X, et al. Adoptive transfer of M2 macrophages promotes locomotor recovery in adult rats after spinal cord injury. Brain Behav Immun. 45, 157–170 (2015).
Jones LAT, Lammertse DP, Charlifue SB, et al. A phase 2 autologous cellular therapy trial in patients with acute, complete spinal cord injury: pragmatics, recruitment, and demographics. Spinal Cord. 48(11), 798–807 (2010).
Knoller N, Auerbach G, Fulga V, et al. Clinical experience using incubated autologous macrophages as a treatment for complete spinal cord injury: phase I study results. J Neurosurg Spine. 3(3), 173–181 (2005).
Lammertse DP, Jones LAT, Charlifue SB, et al. Autologous incubated macrophage therapy in acute, complete spinal cord injury: results of the phase 2 randomized controlled multicenter trial. Spinal Cord. 50(9), 661–671 (2012).
Lammertse DP. Clinical trials in spinal cord injury: lessons learned on the path to translation. The 2011 International Spinal Cord Society Sir Ludwig Guttmann Lecture. Spinal Cord. 51(1), 2–9 (2013).
Kong X, Gao J. Macrophage polarization: a key event in the secondary phase of acute spinal cord injury. J. Cell. Mol. Med. 21(5), 941–954 (2017).
Cheng Z, Zhu W, Cao K, et al. Anti-inflammatory mechanism of neural stem cell transplantation in spinal cord injury. Int J Mol Sci. 17(9) (2016).
Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 3(1), 23–35 (2003).
Francos-Quijorna I, Amo-Aparicio J, Martinez-Muriana A, López-Vales R. IL-4 drives microglia and macrophages toward a phenotype conducive for tissue repair and functional recovery after spinal cord injury. Glia. 64(12), 2079–2092 (2016).
Lima R, Monteiro S, Lopes JP, et al. Systemic interleukin-4 administration after spinal cord injury modulates inflammation and promotes neuroprotection. Pharmaceuticals (Basel). 10(4) (2017).
Coll-Miró M, Francos-Quijorna I, Santos-Nogueira E, et al. Beneficial effects of IL-37 after spinal cord injury in mice. Proc Natl Acad Sci USA. (2016).
Dooley D, Lemmens E, Vangansewinkel T, et al. Cell-based delivery of interleukin-13 directs alternative activation of macrophages resulting in improved functional outcome after spinal cord injury. Stem Cell Reports. 7(6), 1099–1115 (2016).
Guo Y, Zhang H, Yang J, et al. Granulocyte colony-stimulating factor improves alternative activation of microglia under microenvironment of spinal cord injury. Neuroscience. 238, 1–10 (2013).
Dooley D, Lemmens E, Ponsaerts P, Hendrix S. Interleukin-25 is detrimental for recovery after spinal cord injury in mice. J Neuroinflammation. 13(1), 101 (2016).
Guerrero AR, Uchida K, Nakajima H, et al. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J Neuroinflammation. 9, 40 (2012).
Mukaino M, Nakamura M, Yamada O, et al. Anti-IL-6-receptor antibody promotes repair of spinal cord injury by inducing microglia-dominant inflammation. Exp Neurol. 224(2), 403–414 (2010).
Okada S, Nakamura M, Mikami Y, et al. Blockade of interleukin-6 receptor suppresses reactive astrogliosis and ameliorates functional recovery in experimental spinal cord injury. J Neurosci Res. 76(2), 265–276 (2004).
Esposito E, Cuzzocrea S. Anti-TNF therapy in the injured spinal cord. Trends in Pharmacological Sciences. 32(2), 107–115 (2011).
Saxena T, Loomis KH, Pai SB, et al. Nanocarrier-mediated inhibition of macrophage migration inhibitory factor attenuates secondary injury after spinal cord injury. ACS Nano. 9(2), 1492–1505 (2015).
Papa S, Caron I, Erba E, et al. Early modulation of pro-inflammatory microglia by minocycline loaded nanoparticles confers long lasting protection after spinal cord injury. Biomaterials. 75, 13–24 (2016).
Francos-Quijorna I, Santos-Nogueira E, Gronert K, et al. Maresin 1 promotes inflammatory resolution, neuroprotection, and functional neurological recovery after spinal cord injury. Journal of Neuroscience. 37(48), 11731–11743 (2017).
Zhang P, Holscher C, Ma X. Therapeutic potential of flavonoids in spinal cord injury. Rev Neurosci. 28(1), 87–101 (2017).
Ulndreaj A, Chio JCT, Ahuja CS, Fehlings MG. Modulating the immune response in spinal cord injury. Expert Rev Neurother. 16(10), 1127–1129 (2016).
Orr MB, Simkin J, Bailey WM, et al. Compression decreases anatomical and functional recovery and alters inflammation after contusive spinal cord injury. J Neurotrauma. 34(15), 2342–2352 (2017).
Orr MB, Gensel JC. Interactions of primary insult biomechanics and secondary cascades in spinal cord injury: implications for therapy. Neural Regen Res. 12(10), 1618–1619 (2017).
Acknowledgments
This work is supported by NIH R01 NS091582. Stipend support for MO from the Kentucky Spinal Cord and Head Injury Research Trust and the University of Kentucky College of Medicine Fellowship for Excellence in Graduate Research. The authors would like to thank Phillip Popovich and the editors, Mar Cortes, Keith Tansey, and Guillermo Garcia-Alias, for their endorsements.
Required Author Forms
Disclosure forms provided by the authors are available with the online version of this article.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(PDF 1225 kb)
Rights and permissions
About this article
Cite this article
Orr, M.B., Gensel, J.C. Spinal Cord Injury Scarring and Inflammation: Therapies Targeting Glial and Inflammatory Responses. Neurotherapeutics 15, 541–553 (2018). https://doi.org/10.1007/s13311-018-0631-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13311-018-0631-6