Abstract
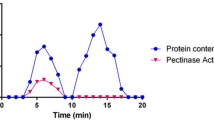
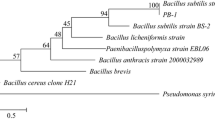
In this paper, we report cloning of a pectate lyase gene from Bacillus amyloliquefaciens S6 (pelS6), and biochemical characterization of the recombinant pectate lyase. PelS6 was found to be identical with B. subtilis 168 pel enzyme with 100% amino acid sequence homology. Although these two are genetically very close, they are distinctly different in physiology. pelS6 gene encodes a 421-aa protein with a molecular mass of 65,75 kDa. Enzyme activity increased from 12.8 ± 0.3 to 49.6 ± 0.4 units/mg after cloning. The relative enzyme activity of the recPel S6 ranged from 80% to 100% at pH between 4 and 14. It was quite stable at different temperature values ranging from 15 to 90 °C. The recPEL S6 showed a maximal activity at pH 10 and at 60 °C. 0.5 mM of CaCl2 is the most effective metal ion on the recPEL S6 as demonstrated by its increased relative activity with 473%. recPEL S6 remained stable at − 20 °C for 18 months. In addition recPEL S6 increased juice clarity. This study introduces a novel bacterial pectate lyase enzyme with its characteristic capability of being highly thermostable, thermotolerant, and active over a wide range of pH, meaning that it can work at both acidic and alkaline environments, which are the most preferred properties in the industry.





Similar content being viewed by others
References
Abu-Qarn, M., Eichler, J., & Sharon, N. (2008). Not just for Eukarya anymore: protein glycosylation in Bacteria and Archaea. Current Opinion in Structural Biology, 18, 544–550.
Alcaraz, L. D., Moreno-Hagelsieb, G., Eguiarte, L. E., et al. (2010). Understanding the evolutionary relationships and major traits of Bacillus through comparative genomics. BMC Genomics, 11, 332.
Biz, A., Farias, F. C., Motter, F. A., et al. (2014). Pectinase activity determination: An early deceleration in the release of reducing sugars throws a spanner in the works! PLoS ONE, 9, e109529.
Bolotin, A., & Borchert, S. (1997). The complete genome sequence of the Gram-positive bacterium Bacillus subtilis. Nature, 390, 249–256. https://doi.org/10.1021/ic00220a054.
Bonnin, E., Ralet, M.-C., Thibault, J.-F., & Schols, H. A. (2009). Enzymes for the valorisation of fruit-and vegetable-based co-products. Handbook of waste management and co-product recovery in food processing (pp. 257–285). Cambridge: Woodhead Publishing.
Bradford, M. M. (1976). A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254. https://doi.org/10.1016/0003-2697(76)90527-3.
Cheng, Z., Chen, D., Lu, B., et al. (2016). A novel acid-stable endo-polygalacturonase from Penicillium oxalicum CZ1028: Purification, characterization, and application in the beverage industry. Journal of Microbiology and Biotechnology, 26, 989–998.
Crawford, M. S., & Kolattukudy, P. E. (1987). Pectate lyase from Fusarium solani f. sp. pisi: Purification, characterization, in vitro translation of the mRNA, and involvement in pathogenicity. Archives of Biochemistry and Biophysics, 258, 196–205.
De Lima Damásio, A. R., Maller, A., Márcio Da Silva, T., et al. (2011). Biotechnological potential of alternative carbon sources for production of pectinases by rhizopus microsporus var. rhizopodiformis. Archives of Biology and Technology, 54154, 141–148.
Dubey, A. K., Yadav, S., Kumar, M., et al. (2016). Molecular biology of microbial pectate lyase: A review. British Biotechnology Journal, 13, 1–26.
Fuchs, A. (1965). The trans-eliminative breakdown of Na-polygalacturonate by Pseudomonas fluorescens. Antonie van Leeuwenhoek, 31, 323–340.
Fujiwara, S. (2002). Extremophiles: Developments of their special functions and potential resources. ShinsukeFujiwara, 94, 518–525.
Garg, G., Singh, A., Kaur, A., et al. (2016). Microbial pectinases an ecofriendly tool of nature for industries. 3 Biotech, 6, 47.
Garron, M. L., & Cygler, M. (2010). Structural and mechanistic classification of uronic acid-containing polysaccharide lyases. Glycobiology, 20, 1547–1573. https://doi.org/10.1093/glycob/cwq122.
Gómez-Plaza, E., Gil-Muñ Oz, R., López-Roca, J. M., & Martínez, A. (2000). Color and phenolic compounds of a young red wine. Influence of wine-making techniques, storage temperature, and length of storage time. Journal of Agriculture and Food Chemistry, 48, 736–741.
Gummadi, S. N., & Panda, T. (2003). Purification and biochemical properties of microbial pectinases—A review. Process Biochemistry, 38, 987–996.
Haki, G. D., & Rakshit, S. K. (2003). Developments in industrially important thermostable enzymes: A review. Bioresource Technology, 89, 17–34.
Hoondal, G., Tiwari, R., Tewari, R., et al. (2002). Microbial alkaline pectinases and their industrial applications: A review. Applied Microbiology and Biotechnology, 59, 409–418.
Hugouvieux-Cotte-Pattat, N., Condemine, G., Nasser, W., & Reverchon, S. (1996). Regulation of pectinolysis in Erwinia chrysanthemi. Annual Review of Microbiology, 50, 213–257.
Hugouvieux-Cotte-Pattat, N., Condemine, G., & Shevchik, V. E. (2014). Bacterial pectate lyases, structural and functional diversity. Environmental Microbiology Reports, 6, 427–440.
Jacob, N. (2009). Biotechnology for agro-industrial residues utilisation: Utilisation of agro-residues. In P. S. Nigam & A. Pandey (Eds.), Biotechnology for agro-industrial residues utilisation: Utilisation of agro-residues (pp. 1–466). Dordrecht: Springer.
Jurick, W. M., Vico, I., McEvoy, J. L., et al. (2009). Isolation, purification, and characterization of a polygalacturonase produced in Penicillium solitum-decayed ‘golden delicious’ apple fruit wayne. Phytopathology, 99, 636–641.
Kashyap, D. R., Vohra, P. K., Chopra, S., & Tewari, R. (2001). Applications of pectinases in the commercial sector: A review. Bioresource Technology, 77, 215–227.
Kita, N., Boyd, C. M., Garrett, M. R., et al. (1996). Differential effect of site-directed mutations in pelC on pectate lyase activity, plant tissue maceration, and elicitor activity. Journal of Biological Chemistry, 271, 26529–26535.
Kleerebezem, M., Hols, P., Bernard, E., et al. (2010). The extracellular biology of the lactobacilli. FEMS Microbiology Reviews, 34, 199–230.
Kobayashi, T., Hatada, Y., Higaki, N., et al. (1999). Enzymatic properties and deduced amino acid sequence of a high-alkaline pectate lyase from an alkaliphilic Bacillus isolate. Biochimica et Biophysica Acta, 1427, 145–154.
Kobayashi, T., Koike, K., Yoshimatsu, T., et al. (1999). Purification and Properties of a low-molecular-weight, high-alkaline pectate lyase from an alkaliphilic strain of Bacillus. Bioscience Biotechnology and Biochemistry, 63, 65–72.
Kohli, P., & Gupta, R. (2015). Alkaline pectinases: A review. Biocatalysis and Agricultural Biotechnology, 4, 279–285.
Kozianowski, G., Canganella, F., Rainey, F. A., et al. (1997). Purification and characterization of thermostable pectate-lyases from a newly isolated thermophilic bacterium, Thermoanaerobacter italicus sp. nov. Extremophiles, 1, 171–182.
Lombard, V., Bernard, T., Rancurel, C., et al. (2010). A hierarchical classification of polysaccharide lyases for glycogenomics. Biochemical Journal, 432, 437–444.
Ma, G., Zhu, W., & Liu, Y. (2016). QM/MM studies on the calcium-assisted β-elimination mechanism of pectate lyase from bacillus subtilis. Proteins: Structure, Function, and Bioinformatics, 84, 1606–1615. https://doi.org/10.1002/prot.25103.
Macmillan, J. D., & Vaughn, R. R. (1962). Purification and properties of a polygalacturonic acid-trans-eliminase produced. Biochemistry, 3, 564–572.
Mukhopadhyay, A., Dasgupta, A. K., Chattopadhyay, D., & Chakrabarti, K. (2012). Improvement of thermostability and activity of pectate lyase in the presence of hydroxyapatite nanoparticles. Bioresource Technology, 116, 348–354.
Nasser, W., Awade, A. C., Reverchon, S., & Robert-Baudouy, J. (1993). Pectate lyase from Bacillus subtilis: Molecular characterization of the gene, and properties of the cloned enzyme. FEBS Letters, 335, 319–326.
Nasser, W., Chalet, F., & Robert-Baudouy, J. (1990). Purification and characterization of extracellular pectate lyase from Bacillus subtilis. Biochimie, 72, 689–695.
Pedrolli, D. B., Monteiro, A. C., Gomes, E., & Carmona, E. C. (2009). Pectin and pectinases: Production, characterization and industrial application of microbial pectinolytic enzymes. Open Biotechnology Journal, 3, 9–18.
Phrommao, E., Yongsawatdigul, J., Rodtong, S., & Yamabhai, M. (2011). A novel subtilase with NaCl-activated and oxidant-stable activity from Virgibacillus sp. SK37. BMC Biotechnology, 11, 1–15.
Pickersgill, R., Jenkins, J., Harris, G., et al. (1994). The structure of Bacillus subtilis pectate lyase in complex with calcium. Nature Structural & Molecular Biology, 1, 717–723.
Poondla, V., Bandikari, R., Subramanyam, R., & Reddy Obulam, V. S. (2015). Low temperature active pectinases production by Saccharomyces cerevisiae isolate and their characterization. Biocatalysis and Agricultural Biotechnology, 4, 70–76.
Pušić, T., Tarbuk, A., & Dekanić, T. (2015). Bio-innovation in cotton fabric scouring- acid and neutral pectinases. Fibres & Textiles in Eastern Europe, 23, 98–103.
Sakai, T., Sakamoto, T., Hallaert, J., & Vandamme, E. J. (1993). Pectin, pectinase, and protopectinase: Production, properties, and applications. Advances in Applied Microbiology, 39, 213–294.
Sambrook, J., Russell, D. W. (2006). Isolation of high-molecular-weight DNA from mammalian cells using formamide. In: Press CSHL (ed) Cold Spring Harb Protoc, 3rd edn. Cold Spring Harbor, NY
Sato, M., & Kaji, A. (1975). Purification and properties of pectate lyase produced by Streptomyces fradiae IFO 3439. Agricultural and Biological Chemistry, 39, 819–824.
Sharma, H. P., & Patel, H. (2017). Critical reviews in food science and nutrition enzymatic added extraction and clarification of fruit juices—A review enzymatic added extraction and clarification of fruit juices—A review. Critical Reviews in Food Science and Nutrition, 57, 1215–1227.
Sharma, R., Lee, D.-W., Xu, Z., et al. (2016). Comparative genomic analysis of Bacillus amyloliquefaciens and Bacillus subtilis reveals evolutional traits for adaptation to plant-associated habitats. Frontiers in Microbiology, 7, 2039.
Sharon, N. (2007). Celebrating the golden anniversary of the discovery of bacillosamine, the diamino sugar of a Bacillus. Glycobiology, 17, 1150–1155.
Singh Jayani, R., Saxena, S., & Gupta, R. (2005). Microbial pectinolytic enzymes: A review. Process Biochemistry, 40, 2931–2944.
Singh, S. A., Plattner, H., & Diekmann, H. (1999). Exopolygalacturonate lyase from a thermophilic Bacillus sp. Enyzme and Microbial Technology, 25, 420–425.
Soriano, M., Blanco, A., Dıaz, P., & Pastor, F. I. J. (2000). An unusual pectate lyase from a Bacillus sp. with high activity on pectin: Cloning and characterization. Microbiology, 146, 89–95.
Soriano, M., Diaz, P., Javier, F. I., et al. (2006). Pectate lyase C from Bacillus subtilis: A novel endo-cleaving enzyme with activity on highly methylated pectin. Microbiology, 152, 617–625.
Starr, M. P., & Moran, F. (1962). Eliminative split of pectic substances by phytopathogenic soft-rot bacteria. Science, 135, 920–921.
Takao, M., Nakaniwa, T., Yoshikawa, K., et al. (2000). Purification and characterization of thermostable pectate lyase with protopectinase activity from thermophilic Bacillus sp. TS 47. Bioscience, Biotechnology, and Biochemistry, 64, 2360–2367.
Van den Burg, B. (2003). Extremophiles as a source for novel enzymes. Current Opinion in Microbiology, 6, 213–218.
Wang, X., Lu, Z., Xu, T., et al. (2018). Improving the specific activity and thermo-stability of alkaline pectate lyase from Bacillus subtilis 168 for bioscouring. Biochemical Engineering Journal, 129, 74–83.
Yadav, S., Yadav, P. K., Yadav, D., et al. (2009). Pectin lyase: A review. Process Biochemistry, 44, 1–10.
Yuan, P., Meng, K., Luo, H., et al. (2011). A novel low-temperature active alkaline pectate lyase from Klebsiella sp. Y1 with potential in textile industry. Process Biochemistry, 46, 1921–1926.
Zhang, C., Yao, J., Zhou, C., et al. (2013). The alkaline pectate lyase PEL168 of Bacillus subtilis heterologously expressed in Pichia pastoris is more stable and efficient for degumming ramie fiber. BMC Biotechnology, 13, 1–9.
Zhou, C., Xue, Y., & Ma, Y. (2017). Characterization and overproduction of a thermo-alkaline pectate lyase from alkaliphilic Bacillus licheniformis with potential in ramie degumming. Process Biochemistry, 54, 49–58. https://doi.org/10.1016/j.procbio.2017.01.010.
Zhou, M., Guo, P., Wang, T., et al. (2017). Metagenomic mining pectinolytic microbes and enzymes from an apple pomace-adapted compost microbial community. Biotechnology for Biofuels, 10, 198.
Zhou, Z., Liu, Y., Chang, Z., et al. (2017). Structure-based engineering of a pectate lyase with improved specific activity for ramie degumming. Applied Microbiology and Biotechnology, 101, 2919–2929.
Acknowledgements
This research was supported by the BAP (Project No. 05/2016-25).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bekli, S., Aktas, B., Gencer, D. et al. Biochemical and Molecular Characterizations of a Novel pH- and Temperature-Stable Pectate Lyase from Bacillus amyloliquefaciens S6 for Industrial Application. Mol Biotechnol 61, 681–693 (2019). https://doi.org/10.1007/s12033-019-00194-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-019-00194-2