Abstract
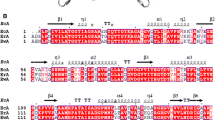
Site-directed mutagenesis of Rhodospirillum rubrum l-asparaginase (RrA) was performed in order to identify sites of the protein molecule important for its therapeutic and physico-chemical properties. Ten multipoint mutant genes were obtained, and five recombinant RrA variants were expressed in E. coli BL21(DE3) cells and isolated as functionally active highly purified proteins. Protein purification was performed using Q-Sepharose and DEAE-Toyopearl chromatography. Overall yield of the active enzymes was 70–80 %, their specific activity at pH 7.4 and 37 °C varied of 140–210 U/mg. l-Glutaminase activity did not exceed 0.01 % of l-asparaginase activity. All RrA mutants showed maximum enzyme activity at pH 9.3–9.5 and 53–58 °C. Km and Vmax values for l-asparagine were evaluated for all mutants. Mutations G86P, D88H, M90K (RrAH), G121L, D123A (RrАI) caused the loss of enzyme activity and confirmed the importance of these sites in the implementation of catalytic functions. Removal of four residues from C-terminal area of the enzyme (RrAK) resulted in the enzyme instability. Mutations D60K, F61L(RrАD), and R118H, G120R(RrАJ) led to the improvement of kinetic parameters and enzyme stabilization. Substitutions E149R, V150P (RrАB) improved antineoplastic and cytotoxic activity of the RrA. A64V, E67K substitutions, especially in combination with E149R, V150P (RrАE), considerably destabilized recombinant enzyme.












Similar content being viewed by others
References
Avramis, V. I., & Tiwari, P. N. (2006). Asparaginase (native ASNase or pegylated ASNase) in the treatment of acute lymphoblastic leukemia. International Journal of Nanomedicine, 1(3), 241–254.
Howard, J. B., & Carpenter, F. H. (1972). l-Asparaginase from Erwinia carotovora. Journal of Biological Chemistry, 247(4), 1020–1030.
Khushoo, A., Pal, Y., & Mukherjee, K. J. (2005). Optimization of extracellular production of recombinant asparaginase in Escherichia coli in shake-flask and bioreactor. Applied Microbiology and Biotechnology, 68(2), 189–197.
Ferrara, M. A., Severino, M. B., Mansure, J. J., Martins, A. S., Oliveira, E. M., Siani, A. C., et al. (2006). Asparaginase production by a recombinant Pichia pastoris strain harbouring Saccharomyces cerevisiae ASP3 gene. Enzyme and Microbial Technology, 39(7), 1457–1463.
Onishi, Y., Yano, S., Thongsanit, J., Takagi, K., Yoshimune, K., & Wakayama, M. (2011). Expression in Escherichia coli of a gene encoding type II l-asparaginase from Bacillus subtilis, and characterization of its unique properties. Annals of Microbiology, 61(3), 517–524.
Schwartz, J. H., Reevesi, J. Y., & Broome, J. D. (1966). Two l-asparaginases from E. coli and their action against tumors. Proceedings of the National Academy of Sciences, 56(5), 1516–1519.
Wink, P. L., Bogdawa, H. M., Renard, G., Chies, J. M., Basso, L. A., & Santos, D. S. (2010). Comparison between two Erwinia carotovora l-asparaginase II constructions: cloning, heterologous expression, purification, and kinetic characterization. Journal of Microbial & Biochemical Technology, 2(1), 13–19.
Duval, M. (2002). Comparison of Escherichia coli asparaginase with Erwinia-asparaginase in the treatment of childhood lymphoid malignancies: results of a randomized European Organisation for Research and Treatment of Cancer-Children’s Leukemia Group phase 3 trials. Blood, 99(8), 2734–2739.
Hawkins, D. S., Park, J. R., Thomson, B. G., Felgenhauer, J. L., Holcenberg, J. S., Panosyan, E. H., et al. (2004). Asparaginase pharmacokinetics after intensive polyethylene glycolconjugated l-asparaginase therapy for children with relapsed acute lymphoblastic leukemia. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research, 10(16), 5335–5341.
Kotzia, G. A., & Labrou, N. E. (2005). Cloning, expression and characterisation of Erwinia carotovora l-asparaginase. Journal of Biotechnology, 119(4), 309–323.
Kotzia, G. A., & Labrou, N. E. (2007). l-Asparaginase from Erwinia chrysanthemi 3937: cloning, expression and characterization. Journal of Biotechnology, 127(4), 657–669.
Palm, G. J., Lubkowski, J., Derst, C., Schleper, S., Röhm, K. H., & Wlodawer, A. (1996). A covalently bound catalytic intermediate in Escherichia coli asparaginase: crystal structure of a Thr-89-Val mutant. FEBS Letters, 390(2), 211–216.
Avramis, V. I., & Panosyan, E. H. (2005). Pharmacokinetic/Pharmacodynamic relations of asparaginase formulations the past, the present and recommendations for the future. Clinical Pharmacokinetics, 44(4), 367–393.
Pui, C. H., Burghen, G. A., Bowman, W. P., & Aur, R. J. (1981). Risk factors for hyperglycemia in children with leukemia receiving l-asparaginase and prednisone. Journal of Pediatrics, 99(1), 46–50.
Evans, W. E., Tsiatis, A., Rivera, G., Murphy, S. B., Dahl, G. V., Denison, M., et al. (1982). Anaphylactoid reactions to Escherichia coli and Erwinia asparaginase in children with leukemia and lymphoma. Cancer, 49(7), 1378–1383.
Priest, J. R., Ramsay, N. K., Steinherz, P. G., Tubergen, D. G., Cairo, M. S., Sitarz, A. L., et al. (1982). A syndrome of thrombosis and hemorrhage complicating l-asparaginase therapy for childhood acute lymphoblastic leukemia. Journal of Pediatrics, 100(6), 984–989.
Sahu, S., Saika, S., Pai, S. K., & Advani, S. H. (1998). l-asparaginase (Leunase) induced pancreatitis in childhood acute lymphoblastic leukemia. Journal of Pediatric Hematology/oncology, 15(6), 533–538.
Feinberg, W. M., & Swenson, M. R. (1988). Cerebrovascular complications of l-asparaginase therapy. Neurology, 38(1), 127–133.
Vrooman, L. M., Supko, J. G., Neuberg, D. S., Asselin, B. L., Athale, U. H., Clavell, L., et al. (2010). Erwinia asparaginase after allergy to E. coli asparaginase in children with acute lymphoblastic leukemia. Pediatric Blood & Cancer, 54(2), 199–205.
Derst, C., Henseling, J., & Röhm, K. H. (2000). Engineering the substrate specificity of Escherichia coli asparaginase II. Selective reduction of glutaminase activity by amino acid replacements at position 248. Protein Science, 9(10), 2009–2017.
Distasio, J. A., Nredrerman, R. A., Kafkewitz, J., & Goodman, D. (1976). Purification and characterization of l-asparaginase with anti-lymphoma activity from Vibrio succinogenes. Journal of Biological Chemistry, 251(22), 6929–6933.
Gladilina, Iu A, Sokolov, N. N., & Krasotkina, Iu V. (2009). Cloning, expression and purification of Helicobater pylori l-asparaginase. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry, 3(1), 89–91.
Mahajan, R. V., Kumar, V., Rajendran, V., Saran, S., Ghosh, P. C., & Saxena, Kumar R. (2014). Purification and characterization of a novel and robust l-asparaginase having Low-glutaminase activity from Bacillus licheniformis: in vitro evaluation of anti-cancerous properties. PlosOne, 9(6), 1–8.
Bansal, S., Gnaneswari, D., Mishra, P., & Kundu, B. (2010). Structural stability and functional analysis of l-asparaginase from Pyrococcus furiosus. Biochemistry (Moscow), 75(3), 375–381.
Pokrovskaya, M. V., Pokrovskiy, V. S., Aleksandrova, S. S., Anisimova, N Iu, Adrianov, R. M., Treshchalina, E. M., et al. (2012). Recombinant intracellular Rhodospirillum rubrum l-asparaginase with low l-glutaminase activity and antiproliferative effect. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry, 6(2), 123–131.
Mezentsev, Yu V, Molnar, A. A., Gnedenko, O. V., Krasotkina, Yu V, Sokolov, N. N., & Ivanov, A. S. (2007). Oligomerization of l-asparaginase from Erwinia carotovora. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry, 1(1), 58–67.
Aghaiypour, K., Wlodawer, A., & Lubkowski, J. (2001). Do bacterial l-asparaginases utilize a catalytic triad Thr-Tyr-Glu? Biochimica et Biophysica Acta, 1550(2), 117–128.
Aung, H. P., Bocola, M., Schleper, S., & Röhm, K. H. (2000). Dynamics of a mobile loop at the active site of Escherichia coli asparaginase. Biochimica et Biophysica Acta, 1481(2), 349–359.
Prakasham, R. S., Hymavathi, M., Rao, C. S., Arepalli, S. K., Rao, J. V., et al. (2010). Evaluation of antineoplastic activity of extracellular asparaginase produced by isolated Bacillus circulans. Applied Biochemistry and Biotechnology, 160(1), 72–80.
Edelheit, O., Hanukoglu, A., & Hanukoglu, I. (2009). Simple and efficient site-directed mutagenesis using two single-primer reactions in parallel to generate mutants for protein structure-function studies. BMC Biotechnology, 9(61), 1–8.
Green, M. R., Sambrook, J., & Peter, MacCallum (2012). Molecular cloning: a laboratory manual (4th Edn), Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press ISBN 978-1-936113-41-5.
Sanger, F., Nicklen, S., & Coulson, A. R. (1977). DNA sequencing with chain-terminating inhibitors. Proceedings of the National Academy of Sciences, 74(12), 5463–5467.
Sedmak, J. J., & Grossberg, S. E. (1977). A rapid, sensitive, and versatile assay for protein using coomassie brilliant blue G250. Analitical Biochemistry, 79(1–2), 544–552.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227(5259), 680–685.
Wriston, J. C., & Yellin, T. O. (1973). l-Asparaginase: a review. Advances in Enzymology and Related Areas of Molecular Biology, 39, 185–248.
Wade, H. E., Robinson, H. K., & Philips, B. W. (1971). Asparaginase and glutaminase activities of bacteria. Journal of General Microbiology, 69(3), 299–312.
Dawson, R. M. C., Elliott, D. C., Elliott, W. H., & Jones, K. M. (1986). Data for biochemical research (third edn). OUP, Oxford: Oxford Science Publications. 580.
Jameel, F., Bogner, R., Mauri, F., & Kalonia, D. (1997). Investigation of physicochemical changes to l-asparaginase during freeze-thaw cycling. Journal of Pharmacy and Pharmacology, 49(5), 472–477.
Abakumova, O., Podobed, O. V., Karalkin, P. A., Kondakova, L. I., & Sokolov, N. N. (2012). Antitumor activity of l-asparaginase from Erwinia carotovora against different human and animal leukemic and solid tumours cell lines. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry, 6(4), 306–315.
Abakumova, O., Podobed, O. V., Borisova, A. A., Sidoruk, K. V., Aleksandrova, S. S., Omelyaniuk, N. M., et al. (2009). Antitumor activity of L-asparaginase from Yersinia pseudotuberculosis. Biochemistry (Moscow) Supplement Series B: Biomedical Chemistry, 3(2), 198–201.
Winzor, D. J. (2011). Gel filtration: a means for estimating the molecular mass of proteins. Biochemical Journal, 1, 1–3.
Arnold, K., Bordoli, L., Kopp, J., & Schwede, T. (2006). The SWISS-MODEL workspace: a web-based environment for protein structure homology modeling. Bioinformatics, 22(2), 195–201.
Petersen, T. N., Brunak, S., von Heijne, G., & Nielsen, H. (2011). Signal P 4.0: discriminating signal peptides from transmembrane regions. Nature Methods, 8(10), 785–786.
Liboshi, Y., Papst, P. J., Hunger, S. P., & Terada, N. (1999). l-Asparaginase inhibits the rapamycin-targeted signaling pathway. Biochemical and Biophysical Research Communications, 260(2), 534–539.
Balcao, V. M., Mateo, C., Fernandez, L., Lafuente, R., Malcota, F. X., & Guisan, J. M. (2001). Structural and functional stabilization of l-asparaginase via subunit: immobilization on to highly activated supports. Biotechnology Progress, 17(3), 537–542.
Libinson, G. S., & Mikhalev, A. V. (1976). Relationship between the magnitude of Km and pH for l-asparaginase. Biochemistry (in Russian), 41(1), 149–152.
Wehner, A., Harms, E., Jennings, M. P., Beacham, I. R., Derst, C., Bast, P., et al. (1992). Site-specific mutagenesis of Escherichia coli asparaginase II. None of the three histidine residues is required for catalysis. European Journal of Biochemistry, 208(2), 475–480.
Shifrin, S., Luborsky, S. W., & Grochowski, B. J. (1971). l-Asparaginase from Escherichia coli B. Physicochcmical studies of the dissociation process. Journal of Biological Chemistry, 246, 7708–7714.
Leung-Toung, R., Li, W., Tam, T. F., & Karimian, K. (2002). Thiol-dependent enzymes and their inhibitors: a review. Current Medical Chemistry, 9(9), 979–1002.
Hethey, J., Lai, J., Loutet, S., Morgan, M., & Tang, V. (2002). Effects of Tricine, Glycine and Tris buffers on alkaline phosphatase activity. Journal of Experimental Microbiology and Immunology, 2, 33–38.
Ma, Lan, Thomas, T., Tibbitts, S., & Evan, R. K. (1995). Escherichia coli alkaline phosphatase: X-ray structural studies of a mutant enzyme (His-412 + Asn) at one w of the catalytically important zinc binding sites. Protein Science, 4(8), 1498–1506.
Gervais, D., & Foote, N. (2014). Recombinant deamidated mutants of Erwinia chrysanthemi L-asparaginase have similar or increased activity compared to wild-type enzyme. Molecular Biotechnology, 56(10), 865–877.
Derst, C., Henseling, J., & Rohm, K. H. (1992). Probing the role of threonine and serine residues of E. coli asparaginase II by site-specific mutagenesis. Protein Engineering, 5(8), 785–789.
Schubert, D., Derst, C., & Röhm, K. H. (1996). Abstract. Annual ABRF Meeting: Biomolecular Techniques, San Francisco, CA.
Sanches, M., Krauchenco, S., & Polikarpov, I. (2007). Structure, substrate complexation and reaction mechanism of bacterial asparaginases. Current Chemcial Biology, 1(1), 75–86.
Harms, E., Wehner, A., Aung, H. P., & Rohtn, K. H. (1991). A catalytic role for threonine-I2 of E. coli asparaginase TI as established by sitedirected mutagenesis. FEBS Letters, 285(1,8), 55–58.
Derst, C., Wehner, A., Specht, V., & Rohm, K.-H. (1994). States and functions of tyrosine residues in Escherichia coli asparaginase II. FEBS European Journal of Biochemistry, 224, 533–540.
Laskowski, R. A., MacArthur, M. W., Moss, D. S., & Thornton, J. M. (1993). PROCHECK: a program to check the stereochemical quality of protein structures. Journal of Applied Crystallography, 26(2), 283–291.
Wehner, A., Derst, C., Specht, V., Aung, H. P., & Rohm, K. H. (1994). The catalytic mechanism of Escherichia coli asparaginase II. Hoppe-Seyler’s Z. Physiol. Chem., 375, 108.
Acknowledgments
We express our sincere gratitude to Dr. Mikhail A. Eldarov (Centre “Bioengineering” RAS, Moscow, Russia) for his participation in the discussion, constructive suggestions, useful critique of this research work as well as for help with translation of this article. Dr. Vasiliy N. Lazarev’s (Scientific Research Institute of Physical–Chemical Medicine, Moscow, Russia) valuable support and assistance in DNA sequencing and Prof. Ekaterina Kolesanova’s (Orekhovich Institute of Biomedical Chemistry, Moscow, Russia) help with protein oligomerisation studies are greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pokrovskaya, M.V., Aleksandrova, S.S., Pokrovsky, V.S. et al. Identification of Functional Regions in the Rhodospirillum rubrum l-Asparaginase by Site-Directed Mutagenesis. Mol Biotechnol 57, 251–264 (2015). https://doi.org/10.1007/s12033-014-9819-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-014-9819-0