Abstract
We have employed gene-trap insertional mutagenesis to identify candidate genes whose disruption confer phenotypic resistance to lytic infection, in independent studies using 12 distinct viruses and several different cell lines. Analysis of >2,000 virus-resistant clones revealed >1,000 candidate host genes, approximately 20 % of which were disrupted in clones surviving separate infections with 2–6 viruses. Interestingly, there were 83 instances in which the insertional mutagenesis vector disrupted transcripts encoding H/ACA-class and C/D-class small nucleolar RNAs (SNORAs and SNORDs, respectively). Of these, 79 SNORAs and SNORDs reside within introns of 29 genes (predominantly protein-coding), while 4 appear to be independent transcription units. siRNA studies targeting candidate SNORA/Ds provided independent confirmation of their roles in infection when tested against cowpox virus, Dengue Fever virus, influenza A virus, human rhinovirus 16, herpes simplex virus 2, or respiratory syncytial virus. Significantly, eight of the nine SNORA/Ds targeted with siRNAs enhanced cellular resistance to multiple viruses suggesting widespread involvement of SNORA/Ds in virus–host interactions and/or virus-induced cell death.
Similar content being viewed by others
Introduction
The goal of this study was to discover cellular genes required for viral replication with the aim of developing anti-viral agents. We employed gene-trap insertional mutagenesis, an approach utilizing a promoterless vector that randomly integrates into the host genome, thereby disrupting (trapping) host genes. There is an absolute requirement for a cellular-based promoter to drive expression of a vector-derived selectable marker conferring neomycin resistance. Libraries of gene-trap insertional mutants [1] were used to select for resistance to lytic infection with a variety of viruses. Using this approach, we have identified over 1,000 candidate cellular genes whose disruption confer survival following exposure to otherwise lytic viral infections [2–8], or Clostridium perfringens epsilon toxin [9, 10]. Candidate genes mediating viral infection are identified in surviving clones by sequencing across genomic integration sites with primers annealing to the U3NeoSV2 vector used for insertional mutagenesis.
While protein-encoding genes accounted for over 90 % of the candidate genes identified, the gene-trap insertional mutagenesis vector also inserts into non-protein-encoding genes. These include small nucleolar RNAs (snoRNAs), which are involved in maturation processes of ribosomal RNAs (rRNAs), small nuclear RNAs (snRNAs), transfer RNAs (tRNAs), and messenger RNAs (mRNAs) [11]. Results presented herein suggest the involvement of two novel classes of snoRNAs in viral replication: SNORAs containing conserved H/ACA boxes that participate in nucleotide pseudouridylation, and SNORDs containing C/D boxes that participate in 2′-O-methylation (Fig. 1) [11]. SNORAs and SNORDs function by assembling into respective small nucleolar ribonucleoprotein complexes (snRNPs), and serve as guide RNAs to direct complimentary target RNA nucleotide modification and aid translation (reviewed in [12]). SNORA/Ds also regulate other cellular processes including alternative splicing, mRNA editing [13], and miRNA-like silencing [14]. Interestingly, a recent study utilizing a promoter trap to randomly inactivate cellular alleles revealed that SNORDs 32a, 33, and 35a contribute to stress-induced apoptosis [15]. Potential roles for this novel RNA class in viral replication are considered.
a SNORAs are composed of two nearly complementary hairpin loops and two single stranded regions with conserved H and ACA boxes. The H box sequence is ANANNA, where N can be G, U, A, or C. Bulging within the hairpin loops allow complementary base pairing with target RNA sequences. SNORAs assemble into small nucleolar ribonucleoprotein (snRNP) complexes that catalyze isomerization of the first unpaired uridine in the bulge region to pseudouridine (Ψ). Pseudouridylation occurs 14–16 nucleotides upstream of either the H box, the ACA box, or both. b C/D box-containing SNORDs assemble into snRNPs in complex with complimentary target RNAs, and catalyze 2′-O-ribose methylation. Imperfect copies of the C and D boxes, termed C′ and D′ may be located internally. SNORDs interact with target RNAs via either of the 10–21 nucleotide antisense regions to guide snRNP-catalyzed 2′-O-ribose methylation 5 nucleotides upstream of the D or D′ box
Materials and Methods
Cell Lines and Viruses
Cell lines used for gene-trap studies were obtained from the NIH AIDS Research and Reference Reagent Program (TZM-bl cells) or American Type Culture Collection (ATCC) (HepG2, Hep3B, L, MDCK, and Vero E6). Cowpox virus (CPV, Brighton strain), influenza virus (FLU, A/PR/8/34 strain, H1N1), human rhinovirus 2 (HRV2, HGP strain), human rhinovirus type 16 (HRV16, 11757 strain), poliovirus (PV, Chat strain), and respiratory syncytial virus (RSV, A2 strain) were obtained from the ATCC. Ebola virus (EBO, Zaire species, 1976 Mayinga strain) and Marburg virus (MBG, 1967 Voege strain) were studied in a BSL4 containment facility at the Centers for Disease Control and Prevention (Atlanta, GA, USA). Herpes simplex virus type 1 (HSV1, KA Strain) and type 2 (HSV2, 186 strain) were kindly provided by Drs. David Knipe (Harvard University) and Patricia Spear (Northwestern University), respectively. Dengue Fever Virus type 2 (DFV2, 16681 strain) was a generous gift from Dr. Guey Perng (Emory University). Reovirus type 1 (REO, Lang strain) was initially obtained from Bernard N. Fields. The U3neoSV1 retrovirus shuttle vector [16] was obtained from H. Earl Ruley (Vanderbilt University) and used as an insertional mutagen to prepare gene-trap libraries with parental, virus-sensitive cells as outlined previously [4, 7, 8, 10].
Generation of Cells Lines Resistant to Lytic Viral Infection from Gene-Trap Libraries
Methods detailing the generation of clonal gene-trap library cell lines resisting lytic REO infection (RIE-1 cells), CPV, EBO, HSV2, MBG virus infection (Vero E6 cells), and FLU infection (MDCK cells) have been described previously [2, 4, 5, 7, 8]. Clonal library cells resisting lytic infection were selected in Hep3B cells (DFV2), TZM-bl cells (HRV2 and HRV16), and Vero E6 cells (HSV1, PV, and RSV), using a similar approach. Briefly, gene-trap libraries, each harboring approximately 104 gene entrapment events, were expanded to 80–90 % confluency until approximately 103 daughter cells represented each clone. The indicated cell lines were infected with DFV (MOI = 0.0002), PV (MOI = 0.001), HRV2 or HSV1 (MOI = 0.005), HRV16 (MOI = 0.01), or RSV (MOI = 0.05). Infection proceeded until >90 % of cells were dead (3–7 days), and then the medium was changed every 2–3 days until surviving clones were visible (generally 2–3 weeks). Surviving clones were detatched with trypsin, expanded, and re-infected at a tenfold higher MOI to confirm resistance. Resistant clones showing >70 % survival following re-infection were selected for expansion to identify trapped genes.
U3neoSV1 Shuttle Vector Rescue and Sequencing
Genomic DNAs from clonal virus-resistant cell lines were extracted using a QIAamp DNA Blood Mini kit (QIAGEN, Inc., Valencia, CA, USA). Shuttle vectors and genomic DNA flanking the U3neoSV1 integration site were recovered by restriction enzyme digests of genomic DNA, self-ligation, transformation into Escherichia coli, and sequencing the resultant carbenecillin-resistant plasmids to identify trapped genes, as described [7].
RNA Interference and qRT-PCR Studies
SNORA/Ds were screened by RNAi for functional roles in viral replication against a panel of six viruses (CPV, DFV2, FLU, HRV16, HSV2, and RSV). Inhibition of viral production was determined by measuring viral RNA production in culture supernatants by quantitative real time PCR (qRT-PCR). The same cell lines used for gene-trap studies (Table 1) were used for RNAi screens, with the exception that RNAi screens against FLU were performed in human HepG2 cells instead of canine MDCK cells.
Cells were seeded in 6-well plates (105 cells/well) and transfected in duplicate wells with 50 nM siRNAs (Dharmacon, Inc.) targeting candidate SNORAs or SNORDs, along with relevant negative and positive control siRNAs (n = 2 independent experiments). AllStars Negative Control siRNA (Qiagen, Inc.) or a non-targeting siRNA (Dharmacon, Inc.) served as negative control siRNAs. Negative controls were used to normalize qRT-PCR results to 100 %. siRNAs targeting viral genes were used as positive controls, as follows: D5R and D7R (CPV), PrM (DFV2), PA (FLU), 2C and VP4 (HRV16), UL29 (HSV2), and the P gene (RSV). Duplicate wells were seeded for uninfected and infected controls, lacking transfection reagents. Hep3B, HepG2, and Vero E6 cells were transfected with 50 nM siRNAs using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA), whereas TZM-bl cells were transfected with siRNAs using HiPerfect (Qiagen, Inc.).
Cells were infected at 48 h post-transfection, using MOIs of 1 (cowpox and HSV2), 0.1 (FLU), 0.01 (HRV16 and RSV), or 0.001 (DFV2). Cells were infected for 2 h at either 37 °C (CPV, DFV2, HSV2, FLU, or RSV) or 33 °C (HRV16). Following the 2-h incubation period, cells were washed with PBS and fresh medium was added. At 3 days post-inoculation, culture supernatants were clarified by centrifugation and 200 μL was lysed in preparation for RNA extraction, using an epMotion 5075 Workstation (Eppendorf) and the PureLink 96 Total RNA Purification Kit (Invitrogen). Total RNA was reverse transcribed using random hexamers (Applied Biosystems, Foster City, CA, USA), and qRT-PCR was performed using an Eppendorf Mastercycler RealPlex2 system, with TaqMan assays developed that detect viral DNAs. To generate standards curves, amplicons produced during real time PCR detection of each viral cDNA were cloned into the pCRII vector (Invitrogen). qRT-PCR was performed using freshly prepared standards, serially diluted over eight logs of copy numbers.
Results and Discussion
We observed that 83 candidate SNORA and SNORD genes were potentially disrupted in clonal cell lines surviving viral infection (Table 1). Most candidate SNORA/D genes (79/83) occur as intronic sequences encoded within 29 host genes, although 4 SNORA/Ds: SNORA76, SNORD3B-2, SNORD93, and SNORD104, are monocistronic. As indicated in Table 1, over half of the host genes encoding SNORA/Ds were disrupted at multiple independent integration sites with the insertional mutagen vector U3neoSV1, and/or were identified in cell lines surviving selection with multiple (2–6) independent viruses. Several SNORA/Ds implicated in our gene-trap studies reside within genes that have been previously been shown to be important for viral infection. For example, BAT1 [17], EIF3A [18], HSPA8 [19], RPS11 [19], and RPS8 [19] influence influenza A infection, and HIV infection may depend on RPL10A [20]. These data indirectly suggested that SNORA/Ds might serve as a previously unknown class of cellular RNAs important for viral replication.
To test this hypothesis, we knocked down expression of a variety of SNORA/Ds with siRNA and examined the resulting cells for susceptibility to six different viruses (Table 2). Expression of nine SNORA/Ds encoded within RCC1 (which also encodes the shorter non-coding SNHG3 gene), or SNHG1 were silenced with siRNAs for 2 days prior to infection with either cowpox virus (CPV), Dengue Fever virus (DFV), influenza A (FLU), human rhinovirus 16 (HRV16), herpes simplex virus 2 (HSV2), or respiratory syncytial virus (RSV). Following a 2-h inoculation, cells were washed to remove inocula, and viral production in culture supernatants was measured by quantitative real time PCR using TaqMan assays annealing to viral genomic sequences. SNORA/Ds tested in siRNA screens were found to limit the capacity of viruses to replicate, with, eight out of nine siRNA-treated cells failing to support replication of three of more viruses (Table 2). While a previous study showed that RCC1 supports HSV1 replication [21], we did not observe that silencing RCC1 or the non-coding Small Nucleolar RNA Host Gene 3 (SNHG3) inhibits HSV2 (data not shown), whereas inhibiting expression of the RCC1-encoded SNORA73A did. RCC1 may be alternatively spliced to include some transcripts to contain SNHG3, and these data may be consistent with SNHG3 serving minimal function in viral infection. As has been observed with SNHG2 (GAS5) [22], SNHG1 is a non-coding gene whose intronic SNORDs are stable and well-conserved between human and mouse, unlike their encoded exons [23]. Thus, silencing of SNORDs encoded within SNHG1 may be expected to account for the observed viral inhibition, rather than the associated transcript.
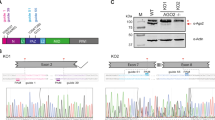
In gene-trap experiments with multiple viruses, RCC1-SNHG3, SNHG2, and TAF1D were the most frequently interrupted genes harboring SNORA/Ds. Gene-trap insertion sites within the RCC1-SNHG3 and SNHG2 loci are shown in Fig. 2. It should be noted that in non-biased selection of clones of murine embryonal stem cells infected with the same insertional vector (data not shown), RCC1-SNHG3 was not found to be a hot spot for insertional events. Therefore, it is unlikely that hot spots for vector insertion is the sole process that lead to 54 independently derived mutant RCC1-SNHG3 clones selected in cells surviving infection with five different viruses (Fig. 2; Table 1). While siRNA confirmation suggest a role for SNORA73A, it may also be considered that the RCC1 gene shares exons with SNHG3, and disrupting SNHG3, perhaps in concert with disrupting SNORA73A, may transmit the resistance phenotype. Interestingly, the prominently represented genes SNHG1, SNHG2, and RCC1-SNHG3 are members of the 5′-terminal oligopyrimidine gene (5′TOP) family [22–24], which contain 4–15 oligopyrimidine tracts in their 5′ ends [25] that regulate transcription and translation under conditions of growth arrest [22, 26]. SHNG2 has recently been found to also be associated with glucocorticoid receptor transcriptional regulation [27], and inhibit cellular proliferation induced by the mammalian target of rapamycin (mTOR) [28]. Thus, there may be processes that work in concert with translational repression that affect viruses capacity for replication. Further work is needed to define whether a role in virus infection is unique for the SHNG genes selected in clones resistant to multiple viruses. Given that SNORDs are encoded within abundantly expressed genes, such as ribosomal genes (Table 1), high expression may be critical for their normal biological roles and in viral infection.
a Gene disruptions within the RCC1, SNHG3, SNORA73A, and SNORA73B loci observed in clonal cell lines resisting lytic viral infection. The SNHG3 gene is a non-coding transcript that shares exons with the protein-coding RCC1 gene (highlighted in green). Introns are designated with black text, whereas SNHG3 exonic sequences not shared with RCC1 are shown in red. SNORA73A and SNORA73B are shown as the first and second intronic sequences highlighted in yellow, respectively. Gene-trap insertions were observed within introns, as well each of the above mentioned genes. Insertion sites are shown with single letters highlighted in blue (human cell lines resisting DFV2, FLU, HRV2, or HRV16 infection) or red (Vero E6 monkey cells resisting HSV2 infection). b SNHG2 and intervening SNORD sequences. Gene-trap insertion sites conferring resistance to pathogens are highlighted in blue text. In some cases, identical clones were recovered from viral selection in independent studies with more than one virus (viruses shown in Table 1). Color coding in 5′ to 3′ orientation: maroon—SNORD44 and SNORD47; light orange—SNORDs 74–81; light blue—SNHG2 coding sequence; black—SNHG2 introns; green—adjacent 5′ and 3′ genomic sequence. Three SNORDs are within coding the sequence for GAS 5, namely SNORDs 47, 76, and 80 (Color figure online)
To our knowledge, this study is the first to show that disrupting or silencing expression of cellular C/D or H/ACA-class small nucleolar RNAs inhibits viral replication. However, emerging data is suggestive of their direct involvement in the process. For example, the Epstein–Barr viral genome encodes a SNORD (v-snoRNA-1), which is thought to target the viral polymerase [29]. In addition, a recent study showed that several small nucleolar RNAs are differentially expressed following severe acute respiratory syndrome coronavirus and influenza virus infection [30]. Indirect evidence suggests possible mechanisms whereby SNORAs and SNORDs may support replication. Several viruses including influenza, HIV-1, HSV2, and adeno-associated virus utilize the nucleolus (the cellular location of SNORAs and SNORDs) during specific stages of their replication cycle [31–34]. Interestingly, SNORAs and SNORDs are known to modify spliceosomal snoRNAs by pseudouridylation or methylation [35–39], which can be important for RNA splicing [40–43], and viral transcript splicing may depend upon host factors [44–48]. Thus, it is possible that SNORAs and SNORDs activate spliceosomal snoRNAs, which promotes the proper splicing and translation of essential viral proteins.
An alternative, though not mutually exclusive, hypothesis is that SNORDs may function in 5′-cap viral RNA maturation through 2′-O-ribose methylation to promote initiation of viral protein translation. In support of this hypothesis are observations that West Nile virus, vesicular stromatitis virus, and Dengue fever virus encode their own 2′-O-methyltransferases that modify 5′-cap structures accordingly, and that methyltransferase mutations are known to impair their replication [49–51]. Influenza A lacks a 2′-O-methyltransferase; however, it utilizes a “cap snatching” mechanism to acquire 5′-caps from cellular mRNAs [52], which are potentially methylated by SNORDs. There are no reports that cellular methyltransferases effect 5′-cap methylation. Interestingly, the 2′-O-methyl group in the cap of cellular mRNAs also strongly influences its ability to act as primer for influenza virus RNA transcription [53].
The discovery of these classes of non-coding genes prominently represented in the mutant clones selected in our virus surviving cell lines suggests an importance of SNORAs and SNORDs in facilitating viral replication. However, this study is not exhaustive in terms of the role each particular SNORA/D may have in viral infection. It is possible that the siRNAs used in our experiments inhibit not only the non-protein encoded RNAs but also the gene in which they reside. Selective in situ mutations in the encoding sequences will need to be performed in order to confirm each as the gene conferring the phenotype. It is anticipated that in future studies, the precise role that SNORAs and SNORDs play will be identified to provide a deeper understanding of the complex interplay at work during the viral-host standoff.
References
von Melchner, H., & Ruley, H. E. (1989). Identification of cellular promoters by using a retrovirus promoter trap. Journal of Virology, 63, 3227–3233.
Dziuba, N., Ferguson, M. R., O’Brien, W. A., Sanchez, A., Prussia, A. J., McDonald, N. J., et al. (2012). Identification of cellular proteins required for replication of human immunodeficiency virus type 1. AIDS Research and Human Retroviruses, 28, 1329–1339.
Friedrich, B. M., Murray, J. L., Guangyu, L., Sheng, J., Hodge, T. W., Rubin, D. H., et al. (2011). A functional role for ADAM10 in human immunodeficiency virus type-1 replication. Retrovirology, 8, 32.
Murray, J. L., Mavrakis, M., McDonald, N. J., Yilla, M., Sheng, J., Bellini, W. J., et al. (2005). Rab9 GTPase is required for replication of human immunodeficiency virus type 1, filoviruses, and measles virus. Journal of Virology, 79, 11742–11751.
Murray, J. L., McDonald, N. J., Sheng, J., Shaw, M. W., Hodge, T. W., Rubin, D. H., et al. (2012). Inhibition of influenza A virus replication by antagonism of a PI3K–AKT–mTOR pathway member identified by gene-trap insertional mutagenesis. Antiviral Chemistry & Chemotherapy, 22, 205–215.
Organ, E. L., Nalbantyan, C. D., Nanney, L. B., Woodward, S. C., Sheng, J., Dubois, R. N., et al. (2004). Effects of transforming growth factor-alpha (TGF-alpha) in vitro and in vivo on reovirus replication. DNA and Cell Biology, 23, 430–441.
Organ, E. L., Sheng, J., Ruley, H. E., & Rubin, D. H. (2004). Discovery of mammalian genes that participate in virus infection. BMC Cell Biology, 5, 41.
Sheng, J., Organ, E. L., Hao, C., Wells, K. S., Ruley, H. E., & Rubin, D. H. (2004). Mutations in the IGF-II pathway that confer resistance to lytic reovirus infection. BMC Cell Biology, 5, 32.
Fennessey, C. M., Sheng, J., Rubin, D. H., & McClain, M. S. (2012). Oligomerization of Clostridium perfringens epsilon toxin is dependent upon caveolins 1 and 2. PLoS ONE, 7, e46866.
Ivie, S. E., Fennessey, C. M., Sheng, J., Rubin, D. H., & McClain, M. S. (2011). Gene-trap mutagenesis identifies mammalian genes contributing to intoxication by Clostridium perfringens epsilon-toxin. PLoS ONE, 6, e17787.
Bachellerie, J. P., Cavaille, J., & Huttenhofer, A. (2002). The expanding snoRNA world. Biochimie, 84, 775–790.
Matera, A. G., Terns, R. M., & Terns, M. P. (2007). Non-coding RNAs: Lessons from the small nuclear and small nucleolar RNAs. Nature Reviews Molecular Cell Biology, 8, 209–220.
Karijolich, J., & Yu, Y. T. (2011). Converting nonsense codons into sense codons by targeted pseudouridylation. Nature, 474, 395–398.
Saraiya, A. A., & Wang, C. C. (2008). snoRNA, a novel precursor of microRNA in Giardia lamblia. PLoS Pathogens, 4, e1000224.
Michel, C. I., Holley, C. L., Scruggs, B. S., Sidhu, R., Brookheart, R. T., Listenberger, L. L., et al. (2011). Small nucleolar RNAs U32a, U33, and U35a are critical mediators of metabolic stress. Cell Metabolism, 14, 33–44.
Hicks, G. G., Shi, E. G., Li, X. M., Li, C. H., Pawlak, M., & Ruley, H. E. (1997). Functional genomics in mice by tagged sequence mutagenesis. Nature Genetics, 16, 338–344.
Momose, F., Basler, C. F., O’Neill, R. E., Iwamatsu, A., Palese, P., & Nagata, K. (2001). Cellular splicing factor RAF-2p48/NPI-5/BAT1/UAP56 interacts with the influenza virus nucleoprotein and enhances viral RNA synthesis. Journal of Virology, 75, 1899–1908.
Karlas, A., Machuy, N., Shin, Y., Pleissner, K. P., Artarini, A., Heuer, D., et al. (2010). Genome-wide RNAi screen identifies human host factors crucial for influenza virus replication. Nature, 463, 818–822.
Hao, L., Sakurai, A., Watanabe, T., Sorensen, E., Nidom, C. A., Newton, M. A., et al. (2008). Drosophila RNAi screen identifies host genes important for influenza virus replication. Nature, 454, 890–893.
Konig, R., Zhou, Y., Elleder, D., Diamond, T. L., Bonamy, G. M., Irelan, J. T., et al. (2008). Global analysis of host-pathogen interactions that regulate early-stage HIV-1 replication. Cell, 135, 49–60.
Strang, B. L., & Stow, N. D. (2007). Blocks to herpes simplex virus type 1 replication in a cell line, tsBN2, encoding a temperature-sensitive RCC1 protein. Journal of General Virology, 88, 376–383.
Smith, C. M., & Steitz, J. A. (1998). Classification of gas5 as a multi-small-nucleolar-RNA (snoRNA) host gene and a member of the 5′-terminal oligopyrimidine gene family reveals common features of snoRNA host genes. Molecular and Cellular Biology, 18, 6897–6909.
Tycowski, K. T., Shu, M. D., & Steitz, J. A. (1996). A mammalian gene with introns instead of exons generating stable RNA products. Nature, 379, 464–466.
Pelczar, P., & Filipowicz, W. (1998). The host gene for intronic U17 small nucleolar RNAs in mammals has no protein-coding potential and is a member of the 5′-terminal oligopyrimidine gene family. Molecular and Cellular Biology, 18, 4509–4518.
Amaldi, F., & Pierandrei-Amaldi, P. (1997). TOP genes: A translationally controlled class of genes including those coding for ribosomal proteins. Progress in Molecular and Subcellular Biology, 18, 1–17.
Yamashita, R., Suzuki, Y., Takeuchi, N., Wakaguri, H., Ueda, T., Sugano, S., et al. (2008). Comprehensive detection of human terminal oligo-pyrimidine (TOP) genes and analysis of their characteristics. Nucleic Acids Research, 36, 3707–3715.
Kino, T., Hurt, D. E., Ichijo, T., Nader, N., & Chrousos, G. P. (2010). Noncoding RNA gas5 is a growth arrest- and starvation-associated repressor of the glucocorticoid receptor. Science Signaling, 3, ra8.
Mourtada-Maarabouni, M., Hasan, A. M., Farzaneh, F., & Williams, G. T. (2010). Inhibition of human T-cell proliferation by mammalian target of rapamycin (mTOR) antagonists requires noncoding RNA growth-arrest-specific transcript 5 (GAS5). Molecular Pharmacology, 78, 19–28.
Hutzinger, R., Feederle, R., Mrazek, J., Schiefermeier, N., Balwierz, P. J., Zavolan, M., et al. (2009). Expression and processing of a small nucleolar RNA from the Epstein–Barr virus genome. PLoS Pathogens, 5, e1000547.
Peng, X., Gralinski, L., Ferris, M. T., Frieman, M. B., Thomas, M. J., Proll, S., et al. (2011). Integrative deep sequencing of the mouse lung transcriptome reveals differential expression of diverse classes of small RNAs in response to respiratory virus infection. MBio, 2, e00198–e00211.
Calle, A., Ugrinova, I., Epstein, A. L., Bouvet, P., Diaz, J. J., & Greco, A. (2008). Nucleolin is required for an efficient herpes simplex virus type 1 infection. Journal of Virology, 82, 4762–4773.
Melen, K., Kinnunen, L., Fagerlund, R., Ikonen, N., Twu, K. Y., Krug, R. M., et al. (2007). Nuclear and nucleolar targeting of influenza A virus NS1 protein: Striking differences between different virus subtypes. Journal of Virology, 81, 5995–6006.
Michienzi, A., Cagnon, L., Bahner, I., & Rossi, J. J. (2000). Ribozyme-mediated inhibition of HIV 1 suggests nucleolar trafficking of HIV-1 RNA. Proceedings of the National Academy of Sciences of the United States of America, 97, 8955–8960.
Ponti, D., Troiano, M., Bellenchi, G. C., Battaglia, P. A., & Gigliani, F. (2008). The HIV Tat protein affects processing of ribosomal RNA precursor. BMC Cell Biology, 9, 32.
Ganot, P., Jady, B. E., Bortolin, M. L., Darzacq, X., & Kiss, T. (1999). Nucleolar factors direct the 2′-O-ribose methylation and pseudouridylation of U6 spliceosomal RNA. Molecular and Cellular Biology, 19, 6906–6917.
Huttenhofer, A., Kiefmann, M., Meier-Ewert, S., O’Brien, J., Lehrach, H., Bachellerie, J. P., et al. (2001). RNomics: An experimental approach that identifies 201 candidates for novel, small, non-messenger RNAs in mouse. EMBO Journal, 20, 2943–2953.
Schattner, P., Barberan-Soler, S., & Lowe, T. M. (2006). A computational screen for mammalian pseudouridylation guide H/ACA RNAs. RNA, 12, 15–25.
Tycowski, K. T., You, Z. H., Graham, P. J., & Steitz, J. A. (1998). Modification of U6 spliceosomal RNA is guided by other small RNAs. Molecular Cell, 2, 629–638.
Vitali, P., Royo, H., Seitz, H., Bachellerie, J. P., Huttenhofer, A., & Cavaille, J. (2003). Identification of 13 novel human modification guide RNAs. Nucleic Acids Research, 31, 6543–6551.
Valadkhan, S., Mohammadi, A., Jaladat, Y., & Geisler, S. (2009). Protein-free small nuclear RNAs catalyze a two-step splicing reaction. Proceedings of the National Academy of Sciences of the United States of America, 106, 11901–11906.
Valadkhan, S., & Manley, J. L. (2009). The use of simple model systems to study spliceosomal catalysis. RNA, 15, 4–7.
Valadkhan, S. (2007). The spliceosome: A ribozyme at heart? Biological Chemistry, 388, 693–697.
Valadkhan, S., & Manley, J. L. (2003). Characterization of the catalytic activity of U2 and U6 snRNAs. RNA, 9, 892–904.
Chiang, C., Chen, G. W., & Shih, S. R. (2008). Mutations at alternative 5′ splice sites of M1 mRNA negatively affect influenza A virus viability and growth rate. Journal of Virology, 82, 10873–10886.
Engelhardt, O. G., & Fodor, E. (2006). Functional association between viral and cellular transcription during influenza virus infection. Reviews in Medical Virology, 16, 329–345.
McLaren, M., Marsh, K., & Cochrane, A. (2008). Modulating HIV-1 RNA processing and utilization. Frontiers in Bioscience, 13, 5693–5707.
Qiu, J., & Pintel, D. (2008). Processing of adeno-associated virus RNA. Frontiers in Bioscience, 13, 3101–3115.
Wagner, E. K., & Bloom, D. C. (1997). Experimental investigation of herpes simplex virus latency. Clinical Microbiology Reviews, 10, 419–443.
Galloway, S. E., Richardson, P. E., & Wertz, G. W. (2008). Analysis of a structural homology model of the 2′-O-ribose methyltransferase domain within the vesicular stomatitis virus L protein. Virology, 382, 69–82.
Kroschewski, H., Lim, S. P., Butcher, R. E., Yap, T. L., Lescar, J., Wright, P. J., et al. (2008). Mutagenesis of the dengue virus type 2 NS5 methyltransferase domain. Journal of Biological Chemistry, 283, 19410–19421.
Zhou, Y., Ray, D., Zhao, Y., Dong, H., Ren, S., Li, Z., et al. (2007). Structure and function of flavivirus NS5 methyltransferase. Journal of Virology, 81, 3891–3903.
Dias, A., Bouvier, D., Crepin, T., McCarthy, A. A., Hart, D. J., Baudin, F., et al. (2009). The cap-snatching endonuclease of influenza virus polymerase resides in the PA subunit. Nature, 458, 914–918.
Bouloy, M., Plotch, S. J., & Krug, R. M. (1980). Both the 7-methyl and the 2′-O-methyl groups in the cap of mRNA strongly influence its ability to act as primer for influenza virus RNA transcription. Proceedings of the National Academy of Sciences of the United States of America, 77, 3952–3956.
Acknowledgments
This work was supported by the Red and Bobby Buisson Foundation, and Public Health Service Small Business Innovation Research (SBIR) Grant AI084705 from the Division of AIDS, National Institute of Allergy and Infectious Diseases. DHR was supported by gifts from Maggie Chassman, the Red and Bobby Buisson Foundation, Inc., Zirus, Inc., and the Public Health Service. None of the funding sources influenced the study design, the collection, analysis of interpretation of data, the preparation of this manuscript, or the decision to submit the article for publication. We also thank Dr. H. Earl Ruley of Vanderbilt University for critical review of the manuscript, and Drs. Natalie McDonald and Thomas Hodge (Zirus, Inc.) for technical support.
Conflict of interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Murray, J.L., Sheng, J. & Rubin, D.H. A Role for H/ACA and C/D Small Nucleolar RNAs in Viral Replication. Mol Biotechnol 56, 429–437 (2014). https://doi.org/10.1007/s12033-013-9730-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-013-9730-0