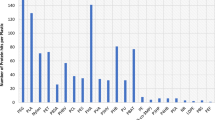
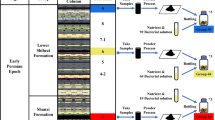
Abstract
The tendency for chlorinated aliphatics and aromatic hydrocarbons to accumulate in environments such as groundwater and sediments poses a serious environmental threat. In this study, the metabolic capacity of hydrocarbon (aromatics and chlorinated aliphatics)-contaminated groundwater in the KwaZulu-Natal province of South Africa has been elucidated for the first time by analysis of pyrosequencing data. The taxonomic data revealed that the metagenomes were dominated by the phylum Proteobacteria (mainly Betaproteobacteria). In addition, Flavobacteriales, Sphingobacteria, Burkholderiales, and Rhodocyclales were the predominant orders present in the individual metagenomes. These orders included microorganisms (Flavobacteria, Dechloromonas aromatica RCB, and Azoarcus) involved in the degradation of aromatic compounds and various other hydrocarbons that were present in the groundwater. Although the metabolic reconstruction of the metagenome represented composite cell networks, the information obtained was sufficient to address questions regarding the metabolic potential of the microbial communities and to correlate the data to the contamination profile of the groundwater. Genes involved in the degradation of benzene and benzoate, heavy metal-resistance mechanisms appeared to provide a survival strategy used by the microbial communities. Analysis of the pyrosequencing-derived data revealed that the metagenomes represent complex microbial communities that have adapted to the geochemical conditions of the groundwater as evidenced by the presence of key enzymes/genes conferring resistance to specific contaminants. Thus, pyrosequencing analysis of the metagenomes provided insights into the microbial activities in hydrocarbon-contaminated habitats.





Similar content being viewed by others
References
Aburto, A., Fahy, A., Coulon, F., Lethbridge, G., Timmis, K., et al. (2009). Mixed aerobic and anaerobic microbial communities in benzene-contaminated groundwater. Journal of Applied Microbiology, 106, 317–328.
Altschul, S. F., Madden, T. L., Schäffer, A. A., Zhang, J., Zhang, Z., et al. (1997). Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Research, 25, 3389–3402.
Beller, H. R., Kane, S. R., Legler, T. C., & Alvarez, P. J. J. (2002). A real-time polymerase chain reaction method for monitoring anaerobic, hydrocarbon-degrading bacteria based on a catabolic gene. Environmental Science and Technology, 36, 3977–3984.
Breitbart, M., Hoare, A., Nitti, A., Siefert, J., Haynes, M., et al. (2009). Metagenomic and stable isotopic analyses of modern freshwater microbialites in Cuatro Cienegas, Mexico. Environmental Microbiology, 11, 16–34.
Brennerova, M. V., Josefiova, J., Brenner, V., Pieper, D. H., & Junca, H. (2009). Metagenomics reveals diversity and abundance of meta-cleavage pathways in microbial communities from soil highly contaminated with jet fuel under air-sparging bioremediation. Environmental Microbiology, 11, 2216–2227.
Bru, D., Sarr, A., & Philippot, L. (2007). Relative abundances of proteobacterial membrane-bound and periplasmic nitrate reductases in selected environments. Applied and Environmental Microbiology, 73, 5971–5974.
Chang, H. Y., Hemp, J., Chen, Y., Fee, J. A., & Gennis, R. B. (2009). The cytochrome ba3 oxygen reductase from Thermus thermophilus uses a single input channel for proton delivery to the active site and for proton pumping. Proceedings of the National Academy of Sciences, 106, 16169–16173.
Chikere, C. B., Okpokwasili, G. C., & Chikere, B. O. (2011). Monitoring of microbial hydrocarbon remediation in the soil. 3 Biotech, 1–22.
Davis, G. B., Patterson, B. M., & Johnston, C. D. (2009). Aerobic bioremediation of 1,2 dichloroethane and vinyl chloride at field scale. Journal of Contaminant Hydrology, 107, 91–100.
Edwards, R., Rodriguez-Brito, B., Wegley, L., Haynes, M., Breitbart, M., et al. (2006). Using pyrosequencing to shed light on deep mine microbial ecology. BMC Genomics, 7, 57.
Gescher, J., Eisenreich, W., Wörth, J., Bacher, A., & Fuchs, G. (2005). Aerobic benzoyl-CoA catabolic pathway in Azoarcus evansii: Studies on the non-oxygenolytic ring cleavage enzyme. Molecular Microbiology, 56, 1586–1600.
Gualandi, G., Frascari, D., Pinelli, D., & Nocentini, M. (2007). Growth of chlorinated solvent-degrading consortia in fed-batch bioreactors and development of a double-substrate high-performing microbial inoculum. Engineering in Life Sciences, 7, 217–228.
Hemalatha, S., & Veeramanikandan, P. (2011). Characterization of aromatic hydrocarbon degrading bacteria from petroleum contaminated sites. Journal of Environmental Protection, 2, 243–254.
Hemme, C. L., Deng, Y., Gentry, T. J., Fields, M. W., Wu, L., et al. (2010). Metagenomic insights into evolution of a heavy metal-contaminated groundwater microbial community. The ISME Journal, 4, 660–672.
Horvath, R. S. (1972). Microbial co-metabolism and the degradation of organic compounds in nature. Bacteriological Reviews, 36, 146.
Huson, D., Richter, D., Mitra, S., Auch, A., & Schuster, S. (2009). Methods for comparative metagenomics. BMC Bioinformatics, 10, S12.
Johnson, D. R., Lee, P. K. H., Holmes, V. F., Fortin, A. C., & Alvarez-Cohen, L. (2005). Transcriptional expression of the tceA gene in a Dehalococcoides-containing microbial enrichment. Applied and Environmental Microbiology, 71, 7145–7151.
Jurelevicius, D., Alvarez, V. M., Peixoto, R., Rosado, A. S., & Seldin, L. (2012). Bacterial polycyclic aromatic hydrocarbon ring-hydroxylating dioxygenases (PAH-RHD) encoding genes in different soils from King George Bay, Antarctic Peninsula. Applied Soil Ecology, 55, 1–9.
Kennedy, J., Flemer, B., Jackson, S. A., Lejon, D. P. H., Morrissey, J. P., et al. (2010). Marine metagenomics: New tools for the study and exploitation of marine microbial metabolism. Marine Drugs, 8, 608–628.
Kim, S. J., Kweon, O., & Cerniglia, C. E. (2009). Proteomic applications to elucidate bacterial aromatic hydrocarbon metabolic pathways. Current Opinion in Microbiology, 12, 301–309.
Krause, L., Diaz, N. N., Goesmann, A., Kelley, S., Nattkemper, T. W., et al. (2008). Phylogenetic classification of short environmental DNA fragments. Nucleic Acids Research, 36, 2230–2239.
Krooneman, J., Sliekers, A. O., Pedro Gomes, T. M., Forney, L. J., & Gottschal, J. C. (2000). Characterization of 3-chlorobenzoate degrading aerobic bacteria isolated under various environmental conditions. FEMS Microbiology Ecology, 32, 53–59.
Mao, J., Luo, Y., Teng, Y., & Li, Z. (2012). Bioremediation of polycyclic aromatic hydrocarbon-contaminated soil by a bacterial consortium and associated microbial community changes. International Biodeterioration and Biodegradation, 70, 141–147.
Margesin, R., & Schinner, F. (2001). Biodegradation and bioremediation of hydrocarbons in extreme environments. Applied Microbiology and Biotechnology, 56, 650–663.
Margulies, M., Egholm, M., Altman, W. E., Attiya, S., Bader, J. S., et al. (2005). Genome sequencing in microfabricated high-density picolitre reactors. Nature, 437, 376–380.
Marzorati, M., Balloi, A., De Ferra, F., Corallo, L., Carpani, G., et al. (2010). Bacterial diversity and reductive dehalogenase redundancy in a 1,2-dichloroethane-degrading bacterial consortium enriched from a contaminated aquifer. Microbial Cell Factories, 9, 12.
Marzorati, M., De Ferra, F., Van Raemdonck, H., Borin, S., Allifranchini, E., et al. (2007). A novel reductive dehalogenase, identified in a contaminated groundwater enrichment culture and in Desulfitobacterium dichloroeliminans strain DCA1, is linked to dehalogenation of 1, 2-dichloroethane. Applied and Environmental Microbiology, 73, 2990–2999.
Meyer, F., Paarmann, D., D’Souza, M., Olson, R., Glass, E. M., et al. (2008). The metagenomics RAST server–a public resource for the automatic phylogenetic and functional analysis of metagenomes. BMC Bioinformatics, 9, 386.
Nemergut, D. R., Robeson, M. S., Kysela, R. F., Martin, A. P., Schmidt, S. K., et al. (2008). Insights and inferences about integron evolution from genomic data. BMC Genomics, 9, 261.
Palmer, J., Oliver, A., & Cameron-Clarke, I. (2001). Containment of groundwater contaminated with chlorinated hydrocarbons, Umbogintwini Industrial Complex, South Africa. Groundwater quality: Natural and enhanced restoration of groundwater pollution, 579–584.
Rees, H. C., Oswald, S. E., Banwart, S. A., Pickup, R. W., & Lerner, D. N. (2007). Biodegradation processes in a laboratory-scale groundwater contaminant plume assessed by fluorescence imaging and microbial analysis. Applied and Environmental Microbiology, 73, 3865–3876.
Salinero, K. K., Keller, K., Feil, W. S., Feil, H., Trong, S., et al. (2009). Metabolic analysis of the soil microbe Dechloromonas aromatica str. RCB: Indications of a surprisingly complex life-style and cryptic anaerobic pathways for aromatic degradation. BMC Genomics, 10, 351.
Sanapareddy, N., Hamp, T. J., Gonzalez, L. C., Hilger, H. A., Fodor, A. A., et al. (2009). Molecular diversity of a North Carolina wastewater treatment plant as revealed by pyrosequencing. Applied and Environmental Microbiology, 75, 1688–1696.
Schmeisser, C., Stöckigt, C., Raasch, C., Wingender, J., Timmis, K., et al. (2003). Metagenome survey of biofilms in drinking-water networks. Applied and Environmental Microbiology, 69, 7298–7309.
Shen, Y., Stehmeier, L. G., & Voordouw, G. (1998). Identification of hydrocarbon-degrading bacteria in soil by reverse sample genome probing. Applied and Environmental Microbiology, 64, 637–645.
Simon, C., Wiezer, A., Strittmatter, A. W., & Daniel, R. (2009). Phylogenetic diversity and metabolic potential revealed in a glacier ice metagenome. Applied and Environmental Microbiology, 75, 7519–7526.
Smith, R. J., Jeffries, T. C., Roudnew, B., Fitch, A. J., Seymour, J. R., et al. (2012). Metagenomic comparison of microbial communities inhabiting confined and unconfined aquifer ecosystems. Environmental Microbiology, 14, 240–253.
Tringe, S. G., Von Mering, C., Kobayashi, A., Salamov, A. A., Chen, K., et al. (2005). Comparative metagenomics of microbial communities. Science, 308, 554–557.
Vaillancourt, F. H., Bolin, J. T., & Eltis, L. D. (2006). The ins and outs of ring-cleaving dioxygenases. Critical Reviews in Biochemistry and Molecular Biology, 41, 241–267.
Weightman, A. J., & Marchesi, J. R. (2003). Comparing the dehalogenase gene pool in cultivated α-halocarboxylic acid-degrading bacteria with the environmental metagene pool. Applied and Environmental Microbiology, 69, 4375–4382.
Yusoff, W. M. W. (2008). Development of three bacteria consortium for the bioremediation of Crude petroleum-oil in contaminated water. OnLine Journal of Biological Sciences, 8, 73–79.
Zaar, A., Gescher, J., Eisenreich, W., Bacher, A., & Fuchs, G. (2004). New enzymes involved in aerobic benzoate metabolism in Azoarcus evansii. Molecular Microbiology, 54, 223–238.
Acknowledgments
The authors wish to thank the National Research Foundation for financial support and TIA (Dr James Sakwa and colleagues) for the pyrosequencing, Dr Rehana Shaik and Dr Algasan Govender for the sample collection and processing.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Abbai, N.S., Pillay, B. Analysis of Hydrocarbon-Contaminated Groundwater Metagenomes as Revealed by High-Throughput Sequencing. Mol Biotechnol 54, 900–912 (2013). https://doi.org/10.1007/s12033-012-9639-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-012-9639-z