Abstract
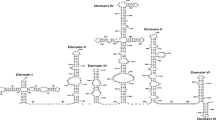
Translation initiation of Coxsackievirus B3 (CVB3) RNA is directed by an internal ribosome entry site (IRES) within the 5′ untranslated region. Host cell factors involved in this process include some canonical translation factors and additional RNA-binding proteins. We have, previously, described that the Sabin3-like mutation (U475 → C) introduced in CVB3 genome led to a defective mutant with a serious reduction in translation efficiency. With the aim to identify proteins interacting with CVB3 wild-type and Sabin3-like IRESes and to study interactions between HeLa cell or BHK-21 protein extracts and CVB3 RNAs, UV-cross-linking assays were performed. We have observed a number of proteins that specifically interact with both RNAs. In particular, molecular weights of five of these proteins resemble to those of the eukaryotic translation initiation factors 4G, 3b, 4B, and PTB. According to cross-linking patterns obtained, we have demonstrated a better affinity of CVB3 RNA binding to BHK-21 proteins and a reduced interaction of the mutant RNA with almost cellular polypeptides compared to the wild-type IRES. On the basis of phylogeny of some initiation factors and on the knowledge of the initiation of translation process, we focused on the interaction of both IRESes with eIF3, p100 (eIF4G), and 40S ribosomal subunit by filter-binding assays. We have demonstrated a better affinity of binding to the wild-type CVB3 IRES. Thus, the reduction efficiency of the mutant RNA to bind to cellular proteins involved in the translation initiation could be the reason behind inefficient IRES function.






Similar content being viewed by others
References
Jackson, R. J., Hellen, C. U. T., & Pestova, T. V. (2010). The mechanism of eukaryotic translation initiation and principles of its regulation. Nature Reviews Molecular Cell Biology, 11, 113–127.
Yu, Y., Abaeva, I. S., Marintchev, A., Pestova, T. V., & Hellen, C. U. T. (2011). Common conformational changes induced in type 2 picornavirus IRESs by cognate trans-acting factors. Nucleic Acids Research, 39, 4851–4865.
Hellen, C. U., & Sarnow, P. (2001). Internal ribosome entry sites in eukaryotic mRNA molecules. Genes & Development, 15, 1593–1612.
Martίnez-Salas, E., Ramos, R., Lafuente, E., & López de Quinto, S. (2001). Functional interactions in internal translation initiation directed by viral and cellular IRES elements. Journal of General Virology, 82, 973–984.
Yang, D., Cheung, P., Sun, Y., Yuan, J., Zhang, H., Carthy, C. M., et al. (2003). A shine-dalgarno-like sequence mediates in vitro ribosomal internal entry and subsequent scanning for translation initiation of Coxsackievirus B3 RNA. Virology, 305, 31–43.
Hernández, G. (2008). Was the initiation of translation in early eukaryotes IRES driven? Trends in Biochemical Sciences, 33, 6–58.
Kieft, J. S. (2008). Viral IRES RNA structures and ribosome interactions. Trends in Biochemical Sciences, 33, 274–283.
Fernández-Miragall, O., López de Quinto, S., & Martínez-Salas, E. (2009). Relevance of RNA structure for the activity of picornavirus IRES elements. Virus Research, 139, 172–182.
Belsham, G. J., & Sonenberg, N. (2000). Picornavirus RNA translation: Roles for cellular proteins. Trends in Microbiology, 8, 330–335.
Martίnez-Salas, E. (2008). The impact of RNA structure on picornavirus IRES activity. Trends in Microbiology, 16, 230–237.
López de Quinto, S., & Martínez-Salas, E. (2000). Interaction of the eIF4G initiation factor with the aphthovirus IRES is essential for internal initiation of translation in vivo. RNA, 6, 1380–1392.
Pilipenko, E. V., Pestova, T. V., Kolupaeva, V. G., Khitrina, E. V., Poperechnaya, A. N., Agol, V. I., et al. (2000). A cell cycle-dependent protein serves as a template-specific translation initiation factor. Genes & Development, 14, 2028–2045.
De Breyne, S., Yu, Y., Pestova, T. V., & Hellen, C. U. T. (2008). Factor requirements for translation initiation on the Simian picornavirus internal ribosomal entry site. RNA, 14, 367–380.
Browning, K. S., Gallie, D. R., Hershey, J. W., Hinnebusch, A. G., Maitra, U., Merrick, W. C., et al. (2001). Unified nomenclature for the subunits of eukaryotic initiation factor 3. Trends in Biochemical Sciences, 26, 284.
Martineau, Y., Derry, M. C., Wang, X., Yanagiya, A., Berlanga, J. J., Shyu, A. B., et al. (2008). Poly (A)-binding protein-interacting protein 1 binds to eukaryotic translation initiation factor 3 to stimulate translation. Molecular and Cellular Biology, 28, 6658–6667.
Hinnebusch, A. G. (2006). eIF3: A versatile scaffold for translation initiation complexes. Trends in Biochemical Sciences, 31, 553–562.
Hershey, J. W. B., & Merrick, W. C. (2000). Pathway and mechanism of initiation of protein synthesis. In N. Sonenberg, J. W. B. Hershey, & M. B. Mathews (Eds.), Translational control of gene expression (pp. 33–88). New York: Cold Spring Harbor Laboratory Press.
López De Quinto, S., Lafuente, E., & Martίnez-Salas, E. (2001). IRES interaction with translation initiation factors: Functional characterization of novel RNA contacts with eIF3, eIF4B, and eIF4GII. RNA, 7, 1213–1226.
Gingras, A. C., Raught, B., & Sonenberg, N. (1999). eIF4 initiation factors: Effectors of mRNA recruitment to ribosomes and regulators of translation. Annual Review of Biochemistry, 68, 913–963.
Nygard, O., & Westermann, P. (1982). Specific interaction of one subunit of eukaryotic initiation factor eIF-3 with 18S ribosomal RNA within the binary complex, eIF-3-small ribosomal subunit, as shown by cross-linking experiments. Nucleic Acids Research, 10, 1327–1334.
Asano, K., Kinzy, T. G., Merrick, W. C., & Hershey, J. W. B. (1997). Conservation and diversity of eukaryotic translation initiation factor eIF3. The Journal of Biological Chemistry, 272, 1101–1109.
Block, K. L., Vornlocher, H. P., & Hershey, J. W. B. (1998). Characterization of cDNAs encoding the p44 and p35 subunits of human translation initiation factor eIF3. The Journal Biological Chemistry, 273, 31901–31908.
Buratti, E., Tisminetzky, S., Zotti, M., & Baralle, F. E. (1998). Functional analysis of the interaction between HCV 5′ UTR and putative subunits of eukaryotic translation initiation factor eIF3. Nucleic Acids Research, 26, 3179–3187.
Sizova, D. V., Kolupaeva, V. G., Pestova, T. V., Shatsky, I. N., & Hellen, C. U. T. (1998). Specific interaction of eukaryotic translation initiation factor 3 with the 5′ nontranslated regions of hepatitis C virus and classical swine fever virus RNAs. Journal of Virology, 72, 4775–4782.
Pestova, T. V., Shatsky, I. N., & Hellen, C. U. T. (1996). Functional dissection of eukaryotic initiation factor eIF4F: The 4A subunit and the central domain of the 4G subunit are sufficient to mediate internal entry of 43S preinitiation complexes. Molecular and Cellular Biology, 16, 6870–6878.
Tarun, S. Z., Jr., & Sachs, A. B. (1996). Association of the yeast poly (A) tail binding protein with translation initiation factor eIF-4G. The EMBO Journal, 15, 7168–7177.
Preiss, T., & Hentze, W. M. (1999). From factors to mechanism: Translation and translational control in eukaryotes. Current Opinion in Genetics & Development, 9, 515–521.
Gradi, A., Imataka, H., Svitkin, Y. V., Rom, E., Raught, B., Morino, S., et al. (1998). A novel functional human eukaryotic translation initiation factor 4G. Molecular and Cellular Biology, 18, 334–342.
Lamphear, B. J., Kirchweger, R., Skern, T., & Rhoads, R. E. (1995). Mapping of functional domains in eukaryotic protein synthesis initiation factor 4G (eIF4G) with picornaviral proteases. The Journal of Biological Chemistry, 270, 21975–21983.
Pulido, M. R., Serrano, P., Sáiz, M., & Martínez-Salas, E. (2007). Foot-and-mouth disease virus infection induces proteolytic cleavage of PTB, eIF3a, b, and PABP RNA-binding proteins. Virology, 364, 466–474.
Imataka, H., Gradi, A., & Sonenberg, N. (1998). A newly identified N-terminal amino acid sequence of human eIF4G binds poly (A)-binding protein and functions in poly (A)-dependent translation. The EMBO Journal, 17, 7480–7489.
Morino, S., Imataka, H., Svitkin, Y. V., Pestova, T. V., & Sonenberg, N. (2000). Eukaryotic translation initiation factor 4E (eIF4E) binding site and the middle one-third of eIF4GI constitute the core domain for cap dependent translation, and the C-terminal one-third functions as a modulatory region. Molecular and Cellular Biology, 20, 468–477.
Korneeva, N. L., Lamphear, B. J., Hennigan, F. L. C., Merrick, W. C., & Rhoads, R. E. (2001). Characterization of the two eIF4A-binding sites on human eIF4G-1. The Journal of Biological Chemistry, 276, 2872–2879.
Imataka, H., & Sonenberg, N. (1997). Human eukaryotic translation initiation factor 4G (eIF4G) possesses two separate and independent binding sites for eIF4A. Molecular and Cellular Biology, 17, 6940–6947.
Korneeva, N. L., Lamphear, B. J., Hennigan, F. L. C., & Rhoads, R. E. (2000). Mutually cooperative binding of eukaryotic translation initiation factor eIF3 and eIF4A to human eIF4G-1. The Journal of Biological Chemistry, 275, 41369–41376.
Ali, I. K., McKendrick, L., Morley, S. J., & Jackson, R. J. (2001). Truncated initiation factor eIF4G lacking an eIF4E binding site can support capped mRNA translation. The EMBO Journal, 20, 4233–4242.
Chau, D. H. W., Yuan, J., Zhang, H., Cheung, P., Lim, T., Liu, Z., et al. (2007). Coxsackievirus B3 proteases 2A and 3C induce apoptotic cell death through mitochondrial injury and cleavage of eIF4GI but not DAP5/p97/NAT1. Apoptosis, 12, 513–524.
Belsham, G. J., & Jackson, R. J. (2000). Translation initiation on picornavirus RNA. In N. Sonenberg, et al. (Eds.), Translational control of gene expression (pp. 869–900). New York: Cold Spring Harbor Laboratory Press.
Stoneley, M., & Willis, A. E. (2004). Cellular internal ribosome entry segments: Structures, trans-acting factors and regulation of gene expression. Oncogene, 23, 3200–3207.
Fernández-Miragall, O., Ramos, R., Ramajo, J., & Martίnez-Salas, E. (2006). Evidence of reciprocal tertiary interactions between conserved motifs involved in organizing RNA structure essential for internal initiation of translation. RNA, 12, 223–234.
Yu, Y., Marintchev, A., Kolupaeva, V. G., Unbehaun, A., Veryasova, T., Lai, S. C., et al. (2009). Position of eukaryotic translation initiation factor eIF1A on the 40S ribosomal subunit mapped by directed hydroxyl radical probing. Nucleic Acid Research, 37, 5167–5182.
Fernández-Miragall, O., & Martίnez-Salas, E. (2003). Structural organization of a viral IRES depends on the integrity of the GNRA motif. RNA, 9, 1333–1344.
Ben M’hadheb-Gharbi, M., Gharbi, J., Paulous, S., Brocard, M., Komaromva, A., Aouni, M., et al. (2006). Effects of the Sabin-like mutations in domain V of the internal ribosome entry segment on translational efficiency of the Coxsackievirus B3. Molecular Genetics and Genomics, 276, 402–412.
Ben M’hadheb-Gharbi, M., Paulous, S., Aouni, M., Kean, K. M., & Gharbi, J. (2007). The substitution U475 C with Sabin3-like mutation within the IRES attenuate coxsackievirus B3 cardiovirulence. Molecular Biotechnology, 36, 52–60.
Ben M’Hadheb-Gharbi, M., El Hiar, R., Paulous, S., Jaidane, H., Aouni, M., Kean, K. M., et al. (2008). Role of GNRA motif mutations within stem–loop V of internal ribosome entry segment in coxsackievirus B3 molecular attenuation. Journal of Molecular Microbiology and Biotechnology, 14, 147–156.
Ben M’hadheb-Gharbi, M., Kean, K. M., & Gharbi, J. (2009). Molecular analysis of the role of IRES stem-loop V in replicative capacities and translation efficiencies of Coxsackievirus B3 mutants. Molecular Biology Reports, 36, 255–262.
Chomczynski, P., & Sacchi, N. (1987). Single-step method of RNA isolation by Acid Guanidium thiocyanate-phenol-Chloroform extraction. Analytical Biochemistry, 162, 156–159.
López de Quinto, S., Saiz, M., de la Morena, D., Sobrino, F., & Martinez-Salas, E. (2002). IRES-driven translation is stimulated separately by the FMDV 30-NCR and poly (A) sequences. Nucleic Acid Research, 30, 4398–4405.
Pisarev, A. V., Unbehaun, A., Hellen, C. U., & Pestova, T. V. (2007). Assembly and analysis of eukaryotic translation initiation complexes. Methods in Enzymology, 430, 147–177.
Locker, N., Chamond, N., & Sargueil, B. (2011). A conserved structure within the HIV gag open reading frame that controls translation initiation directly recruits the 40S subunit and eIF3. Nucleic Acid Research, 39, 2367–2377.
Baboonian, C., Davies, M. J., Booth, J. C., & McKenna, W. J. (1997). Coxsackie B viruses and human heart disease. Current Topics in Microbiology and Immunology, 223, 31–52.
Pasch, A., & Frey, F. J. (2006). Coxsackie B viruses and the kidney: A neglected topic. Nephrology, Dialysis, Transplantation, 21, 1184–1187.
Mena, I., Perry, C. M., Harkin, S., et al. (1999). The role of B lymphocytes in coxsackievirus B3 infection. The American Journal of Pathology, 155, 1205–1215.
Kim, D. S., & Nam, J. H. (2010). Characterization of attenuated coxsackievirus B3 strains and prospects of their application as live-attenuated vaccines. Expert Opinion on Biological Therapy, 10, 179–190.
Johnson, P. R., Feldman, S., Thompson, J. M., et al. (1986). Immunity to influenza A virus infection in young children: A comparison of natural infection, live cold-adapted vaccine, and inactivated vaccine. Journal of Infectious Diseases, 154, 121–127.
Suguitan, A. L., Jr., McAuliffe, J., Mills, K. L., et al. (2006). Live, attenuated influenza A H5N1 candidate vaccines provide broad cross-protection in mice and ferrets. PLoS Medicine, 3, 1541–1555.
Bailey, J. M., & Tapprich, W. E. (2007). Structure of the 5′-nontranslated region of the coxsackievirus B3 genome: Chemical modification and comparative sequence analysis. Journal of Virology, 81, 650–668.
Holcik, M., & Sonenberg, N. (2005). Translational control in stress and apoptosis. Nature Reviews Molecular Cell Biology, 6, 318–327.
Gingras, A. C., & Raught, B. (2007). Signaling to translation initiation. In M. Mathews, N. Sonenberg, & J. W. B. Hershey (Eds.), Translational control in biology and medicine (3rd ed., pp. 369–400). New York: Cold Spring Harbor Laboratory Press.
Brown, B. A., & Ehrenfeld, E. (1979). Translation of poliovirus RNA in vitro: Changes in cleavage pattern and initiation sites by ribosomal salt wash. Virology, 97, 396–405.
Glass, M. J., & Summers, D. F. (1993). Identification of a trans-acting activity from liver that stimulates hepatitis A virus translation in vitro. Virology, 193, 1047–1050.
Stewart, S. R., & Semler, B. L. (1997). RNA determinants of picornavirus cap-independent translation initiation. Seminars in Virology, 8, 242–255.
La Monica, N., & Racaniello, V. R. (1989). Differences in replication of attenuated and neurovirulent polioviruses in human neuroblastoma cell line SH-SY5Y. Journal of Virology, 63, 2357–2360.
Rinehart, J., Gomez, R. M., & Roos, R. P. (1997). Molecular determinants for virulence in coxsackievirus B1 infection. Journal of Virology, 71, 3986–3991.
Tu, Z., Chapman, N., Hufnagel, G., Tracy, S., Romero, J. R., Barry, W. H., et al. (1995). The cardiovirulent phenotype of coxsackievirus B3 is determined at a single site in the genomic 5′nontranslated region. Journal of Virology, 69, 4607–4618.
Yang, D., Wilson, J. E., Anderson, D. R., Bohunek, L., Cordeiro, C., Kandolf, R., et al. (1997). In vitro mutational and inhibitory analysis of the cis-acting translational elements within the 5′ untranslated region of coxsackievirus B3: Potential targets for antiviral action of antisense oligomers. Virology, 228, 63–73.
Dorner, A. J., Semler, B. L., Jackson, R. J., Hanecak, R., Duprey, E., & Wimmer, E. (1984). In vitro translation of poliovirus RNA: Utilization of internal initiation sites in reticulocyte lysate. Journal of Virology, 50, 507–514.
Meerovitch, K., Pelletier, J., & Sonenberg, N. (1989). A cellular protein that binds to the 5′ noncoding region of poliovirus RNA: Implications for internal translation initiation. Genes & Development, 3, 1026–1034.
Ochs, K., Saleh, L., Bassili, G., Sonntag, V. H., Zeller, A., & Niepmann, M. (2002). Interaction of translation initiation factor eIF4B with the poliovirus internal ribosome entry site. Journal of Virology, 76, 2113–2122.
Ochs, K., Zeller, A., Saleh, L., Bassili, G., Song, Y., Sonntag, A., et al. (2003). Impaired binding of standard initiation factors mediates poliovirus translation attenuation. Journal of Virology, 77, 115–122.
Kuge, S., & Nomoto, A. (1987). Construction of viable deletion and insertion mutants of the Sabin strain type 1 poliovirus: Function of the 5′ non coding sequence in viral replication. Journal of Virology, 61, 1478–1487.
Meerovitch, K., Nicholson, R., & Sonenberg, N. (1991). In vitro mutational analysis of cis-acting RNA translational elements within the poliovirus type 2 5′ untranslated region. Journal of Virology, 65, 5895–5901.
Haller, A. A., & Semler, B. L. (1992). Linker scanning mutagenesis of the internal ribosome entry site of poliovirus RNA. Journal of Virology, 66, 5075–5086.
Ehrenfeld, E., & Semler, B. L. (1995). Anatomy of the poliovirus internal ribosome entry site. Current Topics in Microbiology and Immunology, 203, 65–83.
Niepmann, M. (2009). Internal translation initiation of picornaviruses and hepatitis C virus. Biochimica et Biophysica Acta, 1789, 529–541.
Meyer, K., Petersen, A., Niepmann, M., & Beck, E. (1995). Interaction of eukaryotic initiation factor eIF-4B with a picornavirus internal translation initiation site. Journal of Virology, 69, 2819–2824.
Rust, R. C., Ochs, K., Meyer, K., Beck, E., & Niepmann, M. (1999). Interaction of eukaryotic initiation factor eIF4B with the internal ribosome entry site of foot-and-mouth disease virus is independent of the polypyrimidine tract-binding protein. Journal of Virology, 73, 6111–6113.
Méthot, N., Song, M. S., & Sonenberg, N. (1996). A region rich in aspartic acid, arginine, tyrosine, and glycine (DRYG) mediates eukaryotic initiation factor 4B (eIF4B) self-association and interaction with eIF3. Molecular and Cellular Biology, 16, 5328–5334.
Hellen, C. U., Pestova, T. V., Litterst, M., & Wimmer, E. (1994). The cellular polypeptide p57 (pyrimidine tract-binding protein) binds to multiple sites in the poliovirus 5′ nontranslated region. Journal of Virology, 68, 941–950.
Pestova, T. V., Hellen, C. U., & Shatsky, I. N. (1996). Canonical eukaryotic initiation factors determine initiation of translation by internal ribosomal entry. Molecular and Cellular Biology, 16, 6859–6869.
Kolupaeva, V. G., Pestova, T. V., Hellen, C. U., & Shatsky, I. N. (1998). Translation eukaryotic initiation factor 4G recognizes a specific structural element within the internal ribosome entry site of encephalomyocarditis virus RNA. The Journal of Biological Chemistry, 273, 18599–18604.
Kolupaeva, V. G., Lomakin, I. B., Pestova, T. V., & Hellen, C. U. (2003). Eukaryotic initiation factors 4G and 4A mediate conformational changes downstream of the initiation codon of the encephalomyocarditis virus internal ribosomal entry site. Molecular and Cellular Biology, 23, 687–698.
Dildine, S. L., & Semler, B. L. (1992). Conservation of RNA protein interactions among picornaviruses. Journal of Virology, 66, 4364–4376.
Haller, A. A., & Semler, B. L. (1995). Stem-loop structure synergy in binding cellular proteins to the 5′ non-coding region of poliovirus RNA. Virology, 206, 923–934.
Andino, R., Rieckhof, G. E., Achacoso, P. L., & Baltimore, D. (1993). Poliovirus RNA synthesis utilizes an RNP complex formed around the 5′-end of viral RNA. The EMBO Journal, 12, 3587–3598.
Blyn, L. B., Towner, J. S., Semler, B. L., & Ehrenfeld, E. (1997). Requirement of poly (rC) binding protein 2 for translation of poliovirus RNA. Journal of Virology, 71, 6243–6246.
Hellen, C. U., Witherell, G. W., Schmid, M., Shin, S. H., Pestova, T. V., Gil, A., et al. (1993). A cytoplasmic 57-kDa protein that is required for translation of picornavirus RNA by internal ribosomal entry is identical to the nuclear pyrimidine tract-binding protein. Proceedings of the National Academy of Sciences of the United Sciences of America, 90, 7642–7646.
Meerovitch, K., Svitkin, Y. V., Lee, H. S., Lejbkowicz, F., Kenan, D. J., Chan, E. K., et al. (1993). La autoantigen enhances and corrects aberrant translation of poliovirus RNA in reticulocyte lysate. Journal of Virology, 67, 3798–3807.
Ray, P. S., & Das, S. (2002). La autoantigen is required for the internal ribosome entry site-mediated translation of Coxsackievirus B3 RNA. Nucleic Acid Research, 30, 4500–4508.
Bedard, K. M., Walter, B. L., & Semler, B. L. (2004). Multimerization of poly (rC) binding protein 2 is required for translation initiation mediated by a viral IRES. RNA, 10, 1266–1276.
Costa-Mattioli, M., Svitkin, Y., & Sonenberg, N. (2004). La autoantigen is necessary for optimal function of the poliovirus and hepatitis C virus internal ribosome entry site in vivo and in vitro. Molecular and Cellular Biology, 24, 6861–6870.
Yi, M. K., Shultz, D. E., & Lemon, S. M. (2000). Functional significance of the interaction of hepatitis A virus RNA with glyercaldehyde 3-phosphate dehydrogenase (GAPDH): Opposing effects of GAPDH and polypyrimidine tract binding protein on internal ribosomal entry site function. Journal of Virology, 74, 6459–6468.
Schultz, D. E., Hardin, C. C., & Lemon, S. M. (1996). Specific interaction of glyceraldehyde 3-phosphate dehydrogenase with the 5′-nontranslated RNA of hepatitis A virus. The Journal of Biological Chemistry, 271, 14134–14142.
Thomas, A. A., Scheper, G. C., Kleijn, M., De Boer, M., & Voorma, H. O. (1992). Dependence of the adenovirus tripartite leader on the p220 subunit of eukaryotic initiation factor 4F during in vitro translation. Effect of p220 cleavage by foot-and-mouth-disease-virus L-protease on in vitro translation. European Journal of Biochemistry, 207, 471–477.
Merrick, W. C. (1992). Mechanism and regulation of eukaryotic protein synthesis. Microbiological Reviews, 56, 291–315.
Cheung, P., Zhang, M., Yuan, J., Chau, D., Yanagawa, B., McManus, B., et al. (2002). Specific interactions of HeLa cell proteins with coxsackievirus B3 RNA: La autoantigen binds differentially to multiple sites within the 5′untranslated region. Virus Research, 90, 23–36.
Lomakin, I. B., Hellen, C. U. T., & Pestova, T. V. (2000). Physical association of eukaryotic initiation factor 4G (eIF4G) with eIF4A strongly enhances binding of eIF4G to the internal ribosome entry site of encephalomyocarditis virus and is required for internal initiation of translation. Molecular and Cellular Biology, 20, 6019–6029.
Acknowledgments
We gratefully acknowledge all members of the laboratory UMR 8015: “Biological Crystallography and RMN”; Faculty of Pharmacy; University Paris-Descartes (France) and the laboratory of “Molecular Biology”; Research Center “Severo Ochoa”, CSIC; Autonomous University of Madrid (Spain) for providing reagents and scientific technical assistance. The study was supported by research grants from the DGRS/CNRS: “Direction Générale de la Recherche Scientifique, Tunisie” et “le Centre National de la Recherche Scientifique, France” and from a Spanish research project AECI A/018085/08 (Spain).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Souii, A., M’hadheb-Gharbi, M.B., Aouni, M. et al. In Vitro Molecular Characterization of RNA–Proteins Interactions During Initiation of Translation of a Wild-Type and a Mutant Coxsackievirus B3 RNAs. Mol Biotechnol 54, 515–527 (2013). https://doi.org/10.1007/s12033-012-9592-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-012-9592-x