Abstract
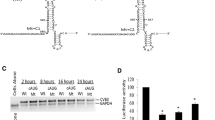
The Sabin3 mutation in the viral RNA plays an important role in directing attenuation phenotype of Sabin vaccine strain of poliovirus type 1 (PV1). We previously described that Sabin3-like mutation introduced in Coxsackievirus B3 (CVB3) genome led to a defective mutant. However, this mutation do not led to destruction of secondary structure motif C within the stem-loop V of CVB3 RNA because of the presence of one nucleotide difference (C → U) in the region encompassing the Sabin3 mutation at nucleotides 471 of PV1 and 475 of CVB3 RNA. In order to reproduce the same sequence of PV1 sabin3 vaccine strain, we introduce in this study an additional mutation (U475 → C) to CVB3 Sabin3-like mutant. Our results demonstrated that Sabin3-like+C mutant displayed a decreased translation initiation defects when translated in cell-free system. This translation initiation defect was correlated with reduced yields of infectious virus particles in HeLa cells in comparison with Sabin3-like mutant and wild-type CVB3 viruses. Inoculation of Swiss mice with mutant viruses resulted in no inflammatory heart disease when compared to heart of mice infected with wild-type. Theses findings indicate that the double mutant could be exploited for the development of a live attenuated vaccine against CVB3.
Similar content being viewed by others
References
Pallansch, M. A., & Roos, R. P. (2001). Enteroviruses: polioviruses, coxsackieviruses, echoviruses, and newer enteroviruses. In␣D. M. Knipe & P. M. Howley (Eds.), Fields Virology. (pp. 723–775). Philadelphia, PA: Lippincott Williams & Wilkins.
Jackson, R. J., & Kaminski, A. (1995). Internal initiation of translation in eukaryotes: the picornavirus paradigm and beyond. RNA, 1, 985–1000.
Liu, Z., Carthy, C. M., Cheung, P., Bohunek, L., Wilson, J. E., McManus, B. M., & Yang, D. (1999). Structural and functional analysis of the 5′ untranslated region of coxsackievirus B3 RNA: In vivo translational and infectivity studies of full-length mutants. Virology, 265, 206–217.
Bhattacharyya, S., & Das, S. (2005). Mapping of secondary structure of the spacer region within the 5′-untranslated region of the coxsackievirus B3 RNA: possible role of an apical GAGA loop in binding La protein and influencing internal initiation of translation. Virus Research, 108, 89–100.
Pelletier, J., Kaplan, G., Racaniello, V. R., & Sonenberg, N. (1988). Cap-independent translation of poliovirus mRNA is conferred by sequence elements within the 5′ noncoding region. Molecular and Cellular Biology, 8, 1103–1112.
Pelletier, J., & Sonenberg, N. (1988). Internal initiation of translation of eukaryotic mRNA directed by a sequence derived from poliovirus RNA. Nature, 334, 320–325.
Baboonian, C., Davies, M. J., Booth, J. C., McKenna, W. J. (1997). Coxsackie B viruses and human heart disease. In S.␣Tracy, N. M. Chapman & B. W. J. Mahy (Eds.), The coxsackie B viruses. (pp. 31–52). Berlin: Springer-Verlag.
Kim, K. S., Hufnagel, G., Chapman, N. M., & Tracy, S. (2001). The group B coxsackieviruses and myocarditis. Reviews in Medical Virology, 11, 355–368.
Galama, J. M. D. (1997). Enteroviral infections in the immunocompromised host. Reviews in Medical Microbiology, 8, 33–40.
Vreugdenhil, G. R., Schloot, N. C., Hoorens, A., Rongen, C., Pipeleers, D. G., Melchers, W. J. G., Roep, B. O., & Galama, J. M. D. (2000). Acute onset of type 1 diabetes mellitus after severe echovirus 9 infection: putative pathogenic pathways. Clinical Infectious Diseases, 31, 1025–1031.
Yoon, J. W., Austin, M., Onodera, T., & Notkins, A. L. (1979). Isolation of a virus from the pancreas of a child with diabetic ketoacidosis. New England Journal of Medicine, 300, 1173–1179.
Gauntt, C. J. (2003). Introduction and historical perspective on experimental myocarditis. In L. T. Cooper Jr. (Ed.), Myocarditis: from Bench to Bedside. (pp. 1–22). Totowa, NJ: Humana Press.
Ramsingh, A. I., Chapman, N., & Tracy, S. (1997). Coxsackieviruses and diabetes. Bioessays, 19, 793–800.
Hyöty, H., & Taylor, K. W. (2002). The role of viruses in human diabetes. Diabetologia, 45, 1353–1361.
Horwitz, M. S., Bradley, L. M., Harbertson, J., Krahl, T., Lee, J., & Sarvetnick, N. (1998). Diabetes induced by Coxsackie virus: initiation by bystander damage and not molecular mimicry. Nature Medicine, 4, 781–785.
See, D. M., & Tilles, J. G. (1995). Pathogenesis of virus-induced diabetes in mice. Journal of Infectious Diseases, 171, 1131–1138.
Tracy, S., Drescher, K. M., Chapman, N. M., Kim, K. S., Carson, S. D., Pirruccello, S., Lane, P. H., Romero, J. R., & Leser, J. S. (2002). Toward testing the hypothesis that group B coxsackieviruses (CVB) trigger insulin-dependent diabetes: inoculating nonobese diabetic mice with CVB markedly lowers diabetes incidence. Journal of Virology, 76, 12097–12111.
Sabin, A. B. (1995). Oral poliovirus vaccine. History of its development and prospects for eradication of poliomyelitis. JAMA, 194, 872–876.
Malnou, C. E., & Kean, K. M. (2003). Translational control of poliovirus gene expression: a seductive model of disease eradication? Current Topics in Virology (in press).
La Monica, N., & Racaniello, V. R. (1989). Differences in replication of attenuated and neurovirulent polioviruses in human neuroblastoma cell line SH-SY5Y. Journal of Virology, 63, 2357–2560.
Macadam, A. J., Ferguson, G., Burlison, J., Stone, D., Skuce, R., Almond, J. W., & Minor, P. D. (1992). Correlation of RNA secondary structure and attenuation of Sabin vaccine strains of poliovirus in tissue culture. Virology, 189, 415–422.
MacAdam, A. J., Pollard, S. R., Ferguson, G., Skuce, R., Wood, D., Almond, J. W., & Minor, P. D. (1993). Genetic basis of attenuation of the Sabin type 2 vaccine strain of poliovirus in primates. Virology, 192, 18–26.
Svitkin, Y. V., Cammack, N., Minor, P. D., & Almond, J. W. (1990). Translation deficiency of the Sabin type 3 poliovirus genome: association with an attenuating mutation C472-U. Virology, 175, 103–109.
Ben M’hadheb-Gharbi, M., Gharbi, J., Paulous, S., Brocard, M., Komarova, A., Aouni, M., & Kean, M. K. (2006). Effects of the Sabin-like mutations in domain V of the internal ribosome entry segment on translational efficiency of the Coxsackievirus B3. Molecular Genetics and Genomics, 276, 402–412.
Teterina, N. L., Kean, M. K., Gorbalenya, A. E., Agol, V. I., & Girard, M. (1992). Analysis of the functional significance of amino acid residues in the putative NTP-binding pattern of the poliovirus 2C protein. The Journal of General Virology, 73, 1977–1986.
Malnou, E. C., Werner, A., Borman, M. A., Westhof, E., & Kean, M. K. (2004). Effects of vaccine strain mutations in domain V of the internal ribosome entry segment compared in the wild type poliovirus type 1 context. The Journal of Biological Chemistry, 279, 10261–10269.
Gharbi, J., El Hiar, R., Ben M’hadheb, M., Jaïdane, H., Bouslama, L., N’saïbia, S., & Aouni, M. (2006). Nucleotide sequences of IRES domains IV and V of natural ECHO virus type 11 isolates with different replication capacity phenotypes. Virus Genes, 32, 269–276.
Jackson, R. J., & Hunt, T. (1983). Preparation and use of nuclease-treated rabbit reticulocyte lysates for the translation of eukaryotic messenger RNA. Methods in Enzymology, 96, 50–74.
Borman, A. M., Deliat, F. G., & Kean, K. M. (1994). Sequences within the poliovirus internal ribosome entry segment control viral RNA synthesis. The EMBO Journal, 13, 3149–3159.
Tu, Z., Chapman, M. N., Hufnagel, G., Tracy, S., Romero, J. R., Barry, W. H., Zhao, L., Currey, K., & Shapiro, B. (1995). The cardiovirulent phenotype of coxsackievirus B3 is determined at a single site in the genomic 5′ nontranslated region. Journal of Virology, 69, 4607–4618.
Zuker, M., Mathews, D. H., & Turner, D. H. (1999). Algorithms and thermodynamics for RNA secondary structure prediction: A practical guide. In J. Barciszewski, & B. F. C. Clark (Eds.), RNA Biochemistry and Biotechnology, NATO ASI Series, Kluwer Academic Publishers.
Agol, V. I. (2002). Picornavirus genome: an overview. In B. L. Semler & E. Wimmer (Eds.), Molecular biology of picornaviruses. (pp. 127–148).Washington DC: ASM Press.
Hellen, C. U., & Wimmer, E. (1995). Enterovirus genetics. In H. A. Rotbart (Ed.), Human Enterovirus Infection (pp. 25–72). Washington DC: American Society for Microbiology.
Gromeier, M., & Nomoto, A. (2002). Determinants of poliovirus pathogenesis. In B. L. Semler, & E. Wimmer (Eds.), Molecular biology of picornavirus (pp. 367–379). Washington DC: ASM Press.
Wimmer, E., Hellen, C. U. T., & Cao, X. M. (1993). Genetics of poliovirus. Annual Review of Genetics, 27, 353–436.
Malnou, C. E., Poyry, T. A. A., Jackson, R. J., & Kean. K. M. (2002). Poliovirus internal ribosome entry segment structure alterations that specifically affect function in neuronal cells: molecular genetic analysis. Journal of Virology, 76, 10617–10626.
Belsham, G. J., & Sonenberg, N. (1996). RNA-protein interactions in regulation of picornavirus RNA translation. Microbiological Reviews, 60, 499–511.
Hunt, S. L., & Jackson, R. J. (1999). Polypyrimidine-tract-binding protein (PTB) is necessary, but not sufficient, for efficient internal initiation of translation of human rhinovirus-2 RNA. RNA, 5, 344–359.
Acknowledgements
We thank Dr. Abdelfattah Zakhama from the college of Medicine of Monastir (Tunisia) for helpful discussion of histology data; Moncef Belayouni and Houda Daami for expert technical assistance. This work was financed by grants to J. Gharbi from the franco-tunisian cooperation: DGRST-CNRS (code 04/R0902) and CMCU (code 04/G0810). M. Ben M’hadheb-Gharbi is supported by CNRS, CMCU and MDT-01 training grants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
M’hadheb-Gharbi, M.B., Paulous, S., Aouni, M. et al. The substitution U475 → C with Sabin3-like mutation within the IRES attenuate Coxsackievirus B3 cardiovirulence. Mol Biotechnol 36, 52–60 (2007). https://doi.org/10.1007/s12033-007-0019-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-007-0019-z