Abstract
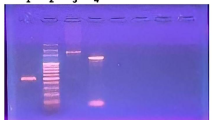
Several investigations are being pursued to enhance the efficacy and specificity of fibrinolytic therapy. In this regard, microbial fibrinolytic enzymes attracted much more medical interests during these decades. Subtilisin, a member of subtilases (the superfamily of subtilisin-like serine proteases) and also a fibrinolytic enzyme is quite common in Gram-positive bacteria, and Bacillus species stand out in particular, as many extracellular and even intracellular variants have been identified. In the present work, the subtilisin gene from Bacillus subtilis PTCC 1023 was cloned into the vector pET-15b and expressed in Escherichia coli strain BL21 (DE3). Total genomic DNA were isolated and used for PCR amplification of the subtilisin gene by means of the specific primers. SDS-PAGE and enzyme assay were done for characterizing the expressed protein. A ~1,100 bp of the structural subtilisin gene was amplified. The DNA and amino acid sequence alignments resulting from the BLAST search of subtilisin showed high sequence identity with the other strains of B. subtilis, whereas significantly lower identity was observed with other bacterial subtilisins. The recombinant enzyme had the same molecular weight as other reported subtilisins and the E. coli transformants showed high subtilisin activity. This study provides evidence that subtilisin can be actively expressed in E. coli. The commercial availability of subtilisin is of great importance for industrial applications and also pharmaceutical purposes as thrombolytic agent. Thus, the characterization of new recombinant subtilisin and the development of rapid, simple, and effective production methods are not only of academic interest, but also of practical importance.




Similar content being viewed by others
References
Peng, Y., Huang, Q., Zhang, R. H., & Zhang, Y. Z. (2003). Purification and characterization of a fibrinolytic enzyme produced by Bacillus amyloliquefaciens DC-4 screened from douchi, a traditional Chinese soybean food. Biochemistry and Physiology Part B: Biochemistry and Molecular Biology, 134, 45–52.
Agrebi, R., Haddar, A., Hmidet, N., Jellouli, K., Manni, L., & Nasri, M. (2009). BSF1 fibrinolytic enzyme from a marine bacterium Bacillus subtilis A26: Purification, biochemical and molecular characterization. Process Biochemistry, 44, 1252–1259.
Hsiao, G., Yen, M., Lee, Y., & Sheu, J. (2002). Antithrombotic effect of PMC, a potent alpha-tocopherol analogue on platelet plug formation in vivo. British Journal Haematology, 117, 699–704.
Ko, J., Yan, J., Zhu, L., & Qi, Y. (2005). Subtilisin QK, a fibrinolytic enzyme, inhibits the exogenous nitrite and hydrogen peroxide induced protein nitration, in vitro and in vivo. Journal of Biochemistry and Molecular Biology, 38(5), 577–583.
Yan, F., Yan, J., Sun, W., Yao, L., Wang, J., Qi, Y., et al. (2009). Thrombolytic effect of Subtilisin QK on carrageenan induced thrombosis model in mice. Journal of Thrombosis and Thrombolysis, 28, 444–448.
Urano, T., Ihara, H., Umemura, K., Suzuki, Y., Oike, M., Akita, S., et al. (2001). The profibrinolytic enzyme subtilisin NAT purified from Bacillus subtilis cleaves and inactivates plasminogen activator inhibitor type 1. Journal of Biological Chemistry, 276(27), 24690–24696.
Sung, J. H., Ahn, S. J., Kim, N. Y., Jeong, S. K., Kim, J. K., Chung, J. K., et al. (2010). Purification, molecular cloning, and biochemical characterization of subtilisin JB1 from a newly isolated Bacillus subtilis JB1. Applied Biochemistry and Biotechnology, 162, 900–911.
Ko, J. H., Yan, J. P., Zhu, L., & Qi, Y. P. (2004). Identification of two novel fibrinolytic enzymes from Bacillus subtilis QK02. Comparative Biochemistry and Physiology Part C, 137, 65.
Xiao, L., Zhang, R. H., Peng, Y., & Zhang, Y. Z. (2004). Highly efficient gene expression of a fibrinolytic enzyme (subtilisin DFE) in Bacillus subtilis mediated by the promoter of α-amylase gene from Bacillus amyloliquefaciens. Biotechnological Letters, 26, 1365–1369.
Zhang, R. H., Xiao, L., Peng, Y., Wang, H. Y., Bai, F., & Zhang, Y. Z. (2005). Gene expression and characteristics of a novel fibrinolytic enzyme (subtilisin DFE) in Escherichia coli. Letters in Applied Microbiology, 41, 190–195.
Weng, M. Z., Zheng, Z. L., Bao, W., Cai, Y. J., Yin, Y., & Zou, G. L. (2009). Enhancement of oxidative stability of the subtilisin nattokinase by site-directed mutagenesis expressed in Escherichia coli. Biochimica et Biophysica Acta, 1794, 1566–1572.
Choi, N. S., Chang, K. T., Maeng, P. J., & Kim, S. H. (2004). Cloning, expression, and fibrin (ogen)olytic properties of a subtilisin DJ-4 gene from Bacillus sp. DJ-4. FEMS Microbiology Letters, 236, 325–331.
Peng, Y., Yang, X. J., Xiao, L., & Zhang, Y. Z. (2004). Cloning and expression of a fibrinolytic enzyme (subtilisin DFE) gene from Bacillus amyloliquefaciens DC-4 in Bacillus subtilis. Research in Microbiology, 155, 167–173.
Chen, Y. J., Wu, K. P., Kim, S., Falzon, L., Inouye, M., & Baum, J. (2008). Backbone NMR assignments of DFP-inhibited mature subtilisin E. Biomolecular NMR Assignments, 2, 131–133.
Fuka, M. M., Engel, M., Haesler, F., Welzl, G., Munch, J. C., & Schloter, M. (2008). Diversity of proteolytic community encoding for subtilisin in an arable field: spatial and temporal variability. Biology and Fertility of Soils, 45, 185–191.
Jang, J. S., Kang, D. O., Park, K. S., & Byun, S. M. (1993). Purification and characterization of recombinant Bacillus stearothermophilus subtilisin. Korean Biochemical Journal, 26(7), 595–601.
Takagi, H., Morinaga, Y., Ikemura, H., & Inouye, M. (1988). Mutant subtilisin E with enhanced protease activity obtained by site-directed mutagenesis. Journal of Biological Chemistry, 263(36), 19592–19596.
Gyowacka, A. E., Podstawka, E., Szczesna-Antczak, M. H., Kalinowska, H., & Antczak, T. (2005). Kinetic and molecular properties of Bacillus subtilis IBTC-3 subtilisin. Comparative Biochemistry and Physiology Part B., 140, 321–331.
Araújo, R., Cavaco-Paulo, A., & Casal, M. (2008). Strategies towards the functionalization of subtilisin E from Bacillus subtilis for wool finishing applications. Engineering in Life Sciences, 8(3), 238–249.
Bryant, M. K., Schardl, C. L., Hesse, U., & Scott, B. (2009). Evolution of a subtilisin-like protease gene family in the grass endophytic fungus Epichloë festucae. BMC Evolutionary Biology, 9, 168–181.
Micheelsen, P. O., Vévodová, J., De Maria, L., Østergaard, P. R., Friis, E. P., Wilson, K., et al. (2008). Structural and mutational analyses of the interaction between the barley α-amylase/subtilisin inhibitor and the subtilisin savinase reveal a novel mode of inhibition. Journal of Molecular Biology, 380, 681–690.
Gololobov, M. Y., Morozova, I. P., Vojushina, T. L., Timokhina, E. A., & Stepanov, V. M. (1992). Subtilisin from Bacillus subtilis strain 72. The influence of substrate structure, temperature and pH on catalytic properties. Biochimica et Biophysica Acta, 1118, 267–276.
Bryan, P. N. (2000). Protein engineering of subtilisin. Biochimica et Biophysica Acta, 1543, 203–222.
Foophow, T., Tanaka, S., Koga, Y., Takano, K., & Kanaya, S. (2010). Subtilisin-like serine protease from hyperthermophilic archaeon Thermococcus kodakaraensis with N- and C-terminal propeptides. Protein Engineering Design and Selection, 23(5), 347–355.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Tamura, Y., Okada, K., Kawao, N., Yano, M., Ueshima, S., Nagai, N., et al. (2011). Profibrinolytic effect of Enzamin, an extract of metabolic products from Bacillus subtilis AK and Lactobacillus. Journal of Thrombosis and Thrombolysis, 24, 550–556.
Higazi, A. A. R., Finci-Yeheskel, Z., Samara, A. A. R., Aziza, R., & Mayer, M. (1992). Stimulation of plasmin activity by oleic acid. Biochemical Journal, 282, 863–866.
Couto, L. T., Donato, J. L., & Nucci, G. D. (2004). Analysis of five streptokinase formulations using the euglobulin lysis test and the plasminogen activation assay. Brazilian Journal of Medicine and Biological Research, 37, 1889–1894.
Humphries, J., Vasudevan, J., & Gonias, S. (1993). Fibrinogenolytic and fibrinolytic activity of cell-associated plasmin. Arteriosclerosis, Thrombosis, and Vascular Biology, 13, 48–55.
Takagi, H., Suzumura, A., Hoshino, T., & Nakamori, S. (1999). Gene expression of Bacillus subtilis subtilisin E in Thermus thermophilus. Journal of Industrial Microbiology & Biotechnology, 23, 214–217.
Jasmin, C., Chellappan, S., Sukumaran, R. K., Elyas, K. K., Bhat, S. G., & Chandrasekaran, M. (2010). Molecular cloning and homology modelling of a subtilisin-like serine protease from the marine fungus, Engyodontium album BTMFS10. World Journal of Microbiology & Biotechnology, 26(7), 1269–1279.
Bhaskar, V., Raj, T. R. J., Kandasamy, S. K. J., Vijaykumar, P., & Achary, A. (2008). Optimization of production of subtilisin in solid substrate fermentation using response surface methodology. African Journal of Biotechnology, 7(13), 2286–2291.
Swartz, J. R. (2001). Advances in Escherichia coli production of therapeutic proteins. Current Opinion in Biotechnology, 12, 195–201.
McPherson, M. J., & Møller, S. G. (2006). In E. Owen (Ed.) PCR (2nd ed.). New York: Taylor & Francis Group, ISBN 0-203-00267-9.
Zhang, H. B., Wang, Y. J., Hu, X. Q., Zhu, H. X., & Wei, Z. J. (2009). Effect of different culture conditions for dextransucrase production in Escherichia coli using lactose as inducer. African Journal of Biotechnology, 8(8), 1577–1582.
Howhan, P., & Pornbanlualap, S. (2003). Cloning and effective induction of Escherichia coli nucleoside diphosphate kinase by lactose. ScienceAsia, 29, 347–353.
Acknowledgments
This study was supported by a grant from the Research Council of Shiraz University of Medical Sciences, Shiraz University of Medical Sciences, Shiraz, Iran.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ghasemi, Y., Dabbagh, F. & Ghasemian, A. Cloning of a Fibrinolytic Enzyme (Subtilisin) Gene From Bacillus subtilis in Escherichia coli . Mol Biotechnol 52, 1–7 (2012). https://doi.org/10.1007/s12033-011-9467-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12033-011-9467-6