Abstract
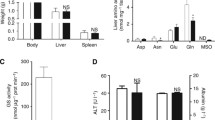
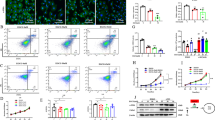
The glutamine synthetase (GS) facilitates cancer cell growth by catalyzing de novo glutamine synthesis. This enzyme removes ammonia waste from the liver following the urea cycle. Since cancer development is associated with dysregulated urea cycles, there has been no investigation of GS’s role in ammonia clearance. Here, we demonstrate that, although GS expression is increased in the setting of β-catenin oncogenic activation, it is insufficient to clear the ammonia waste burden due to the dysregulated urea cycle and may thus be unable to prevent cancer formation. In vivo study, a total of 165 male Swiss albino mice allocated in 11 groups were used, and liver cancer was induced by p-DAB. The activity of GS was evaluated along with the relative expression of mTOR, β-catenin, MMP-14, and GS genes in liver samples and HepG2 cells using qRT-PCR. Moreover, the cytotoxicity of the NH3 scavenger phenyl acetate (PA) and/or GS-inhibitor L-methionine sulfoximine (MSO) and the migratory potential of cells was assessed by MTT and wound healing assays, respectively. The Swiss target prediction algorithm was used to screen the mentioned compounds for probable targets. The treatment of the HepG2 cell line with PA plus MSO demonstrated strong cytotoxicity. The post-scratch remaining wound area (%) in the untreated HepG2 cells was 2.0%. In contrast, the remaining wound area (%) in the cells treated with PA, MSO, and PA + MSO for 48 h was 61.1, 55.8, and 78.5%, respectively. The combination of the two drugs had the greatest effect, resulting in the greatest decrease in the GS activity, β-catenin, and mTOR expression. MSO and PA are both capable of suppressing mTOR, a key player in the development of HCC, and MMP-14, a key player in the development of HCC. PA inhibited the MMP-14 enzyme more effectively than MSO, implying that PA might be a better way to target HCC as it inhibited MMP-14 more effectively than MSO. A large number of abnormal hepatocytes (5%) were found to be present in the HCC mice compared to mice in the control group as determined by the histopathological lesions scores. In contrast, PA, MSO, and PA + MSO showed a significant reduction in the hepatic lesions score either when protecting the liver or when treating the liver. The molecular docking study indicated that PA and MSO form a three-dimensional structure with NF-κB and COX-II, blocking their ability to promote cancer and cause gene mutations. PA and MSO could be used to manipulate GS activities to modulate ammonia levels, thus providing a potential treatment for ammonia homeostasis.












Similar content being viewed by others
Data availability
All data generated or analyzed during this study are included in this published article (and its supplementary information files).
References
Claeys W, et al. A mouse model of hepatic encephalopathy: bile duct ligation induces brain ammonia overload, glial cell activation and neuroinflammation. Sci Rep. 2022;12(1):1–16.
Fiati Kenston SS, Song X, Li Z, Zhao J. Mechanistic insight, diagnosis, and treatment of ammonia-induced hepatic encephalopathy. J Gastroenterol Hepatol. 2019;34(1):31–9.
Limón ID, Angulo-Cruz I, Sánchez-Abdon L, Patricio-Martínez A. Disturbance of the glutamate-glutamine cycle, secondary to hepatic damage, compromises memory function. Front Neurosci. 2021;15: 578922.
Nardelli S, et al. Muscle alterations are associated with minimal and overt hepatic encephalopathy in patients with liver cirrhosis. Hepatology. 2019;70(5):1704–13.
Canbay A, Sowa J-P. L-ornithine L-aspartate (LOLA) as a novel approach for therapy of non-alcoholic fatty liver disease. Drugs. 2019;79(1):39–44.
Zhou Y, Eid T, Hassel B, Danbolt NC. Novel aspects of glutamine synthetase in ammonia homeostasis. Neurochem Int. 2020;140: 104809.
Li X, Zhu H, Sun W, Yang X, Nie Q, Fang X. Role of glutamine and its metabolite ammonia in crosstalk of cancer-associated fibroblasts and cancer cells. Cancer Cell Int. 2021;21(1):1–13.
Hou Y, Hu S, Li X, He W, Wu G. Amino acid metabolism in the liver: nutritional and physiological significance. Amin Acids Nutr Heal. 2020. https://doi.org/10.1007/978-3-030-45328-2_2.
Dai W, et al. Glutamine synthetase limits β-catenin–mutated liver cancer growth by maintaining nitrogen homeostasis and suppressing mTORC1. J Clin Invest. 2022. https://doi.org/10.1172/JCI161408.
J. B. Spinelli, 2019 “Investigating the Fate of Ammonia in Breast Cancer.” Harvard University.
Spinelli JB, Yoon H, Ringel AE, Jeanfavre S, Clish CB, Haigis MC. Metabolic recycling of ammonia via glutamate dehydrogenase supports breast cancer biomass. Science. 2017;80:941–6.
Liu L, Huang Z, Chen J, Wang J, Wang S. Protein phosphatase 2A activation mechanism contributes to JS-K induced caspase-dependent apoptosis in human hepatocellular carcinoma cells. J Exp Clin Cancer Res. 2018;37(1):1–15.
Monga SP. β-catenin signaling and roles in liver homeostasis, injury, and tumorigenesis. Gastroenterology. 2015;148(7):1294–310.
Kim LC, Cook RS, Chen J. mTORC1 and mTORC2 in cancer and the tumor microenvironment. Oncogene. 2017;36(16):2191–201.
Yecies JL, Manning BD. mTOR links oncogenic signaling to tumor cell metabolism. J Mol Med. 2011;89(3):221–8.
Mafi S, et al. mTOR-mediated regulation of immune responses in cancer and tumor microenvironment. Front Immunol. 2022;12:5724.
Henderson N, Markwick LJ, Elshaw SR, Freyer AM, Knox AJ, Johnson SR. Collagen I and thrombin activate MMP-2 by MMP-14-dependent and-independent pathways: implications for airway smooth muscle migration. Am J Physiol Cell Mol Physiol. 2007;292(4):L1030–8.
Alaseem A, Alhazzani K, Dondapati P, Alobid S, Bishayee A, Rathinavelu A. Matrix Metalloproteinases: a challenging paradigm of cancer management. Semin Cancer Biol. 2019;56:100–15.
Angius F, Floris A. Liposomes and MTT cell viability assay: an incompatible affair. Toxicol Vitr. 2015;29(2):314–9.
Pathak S, Kumar Das J, Jyoti Biswas S, Khuda-Bukhsh AR. Protective potentials of a potentized homeopathic drug, Lycopodium-30, in ameliorating azo dye induced hepatocarcinogenesis in mice. Mol Cell Biochem. 2006;285(1):121–31.
Wei MX, Liu JM, Gadal F, Yi P, Liu J, Crepin M. Sodium phenylacetate (NaPa) improves the TAM effect on glioblastoma experimental tumors by inducing cell growth arrest and apoptosis. Anticancer Res. 2007;27(2):953–8.
Chiu M, et al. Glutamine depletion by crisantaspase hinders the growth of human hepatocellular carcinoma xenografts. Br J Cancer. 2014;111(6):1159–67.
Attia AA, et al. Amygdalin potentiates the anti-cancer effect of Sorafenib on Ehrlich ascites carcinoma and ameliorates the associated liver damage. Sci Rep. 2022;12(1):1–9.
Gobe G, Zhang X-J, Willgoss DA, Schoch E, Hogg NA, Endre ZH. Relationship between expression of Bcl-2 genes and growth factors in ischemic acute renal failure in the rat. J Am Soc Nephrol. 2000;11(3):454–67.
S. Martinotti and E. Ranzato, 2019 “Scratch wound healing assay,” in Epidermal cells, Springer. pp. 225–229
Yadollah-Damavandi S, et al. Topical Hypericum perforatum improves tissue regeneration in full-thickness excisional wounds in diabetic rat model evidence-based complement. Altern Med. 2015. https://doi.org/10.1155/2015/245328.
Bancroft JD, Layton C. The hematoxylins and eosin Bancroft’s theory. Pract Histol Tech. 2012;7:173–86.
Gibson-Corley KN, Olivier AK, Meyerholz DK. Principles for valid histopathologic scoring in research. Vet Pathol. 2013;50(6):1007–15.
Holecek M. Evidence of a vicious cycle in glutamine synthesis and breakdown in pathogenesis of hepatic encephalopathy–therapeutic perspectives. Metab Brain Dis. 2014;29(1):9–17.
Matsumoto S, Häberle J, Kido J, Mitsubuchi H, Endo F, Nakamura K. Urea cycle disorders—update. J Hum Genet. 2019;64(9):833–47.
Dang CV. Glutaminolysis: supplying carbon or nitrogen or both for cancer cells? Cell Cycle. 2010;9(19):3884–6.
Kurmi K, Haigis MC. Nitrogen metabolism in cancer and immunity. Trends Cell Biol. 2020;30(5):408–24.
Galadari S, Rahman A, Pallichankandy S, Thayyullathil F. Reactive oxygen species and cancer paradox: to promote or to suppress? Free Radic Biol Med. 2017;104:144–64.
Bigot A, Tchan MC, Thoreau B, Blasco H, Maillot F. Liver involvement in urea cycle disorders: a review of the literature. J Inherit Metab Dis. 2017;40(6):757–69.
Rojas Á, García-Lozano MR, Gil-Gómez A, Romero-Gómez M, Ampuero J. Glutaminolysis-ammonia-urea cycle axis, non-alcoholic fatty liver disease progression and development of novel therapies. J Clin Transl Hepatol. 2022;10(2):356.
Yoo HC, Yu YC, Sung Y, Han JM. Glutamine reliance in cell metabolism. Exp Mol Med. 2020;52(9):1496–516.
Wang B, et al. Glutamine and intestinal barrier function. Amino Acids. 2015;47(10):2143–54.
Jiang J, Srivastava S, Zhang J. Starve cancer cells of glutamine: break the spell or make a hungry monster? Cancers (Basel). 2019;11(6):804.
Ye J, et al. Targeting of glutamine transporter ASCT2 and glutamine synthetase suppresses gastric cancer cell growth. J Cancer Res Clin Oncol. 2018;144(5):821–33.
Kung H-N, Marks JR, Chi J-T. Glutamine synthetase is a genetic determinant of cell type–specific glutamine independence in breast epithelia. PLoS Genet. 2011;7(8): e1002229.
Schlageter M, Terracciano LM, D’Angelo S, Sorrentino P. Histopathology of hepatocellular carcinoma. World J Gastroenterol WJG. 2014;20(43):15955.
Sakamoto M. Early HCC: diagnosis and molecular markers. J Gastroenterol. 2009;44(19):108–11.
Furusawa A, et al. Ovarian cancer therapeutic potential of glutamine depletion based on GS expression. Carcinogenesis. 2018;39(6):758–66.
Li B, et al. Targeting glutaminase 1 attenuates stemness properties in hepatocellular carcinoma by increasing reactive oxygen species and suppressing Wnt/beta-catenin pathway. EBioMedicine. 2019;39:239–54.
Jin H, et al. A powerful drug combination strategy targeting glutamine addiction for the treatment of human liver cancer. Elife. 2020;9: e56749.
Wang C, et al. Inducing and exploiting vulnerabilities for the treatment of liver cancer. Nature. 2019;574(7777):268–72.
Huang X, Gan G, Wang X, Xu T, Xie W. The HGF-MET axis coordinates liver cancer metabolism and autophagy for chemotherapeutic resistance. Autophagy. 2019;15(7):1258–79.
Wang Y, et al. Sirtuin 4 depletion promotes hepatocellular carcinoma tumorigenesis through regulating adenosine-monophosphate–activated protein kinase alpha/mammalian target of rapamycin axis in mice. Hepatology. 2019;69(4):1614–31.
Xiang Y, et al. Targeted inhibition of tumor-specific glutaminase diminishes cell-autonomous tumorigenesis. J Clin Invest. 2015;125(6):2293–306.
Yuan H, et al. RHBDF1 regulates APC-mediated stimulation of the epithelial-to-mesenchymal transition and proliferation of colorectal cancer cells in part via the Wnt/β-catenin signalling pathway. Exp Cell Res. 2018;368(1):24–36.
Schultz GS, Wysocki A. Interactions between extracellular matrix and growth factors in wound healing. Wound repair Regen. 2009;17(2):153–62.
Barrientos S, Stojadinovic O, Golinko MS, Brem H, Tomic-Canic M. Growth factors and cytokines in wound healing. Wound repair Regen. 2008;16(5):585–601.
Santoro MM, Gaudino G. Cellular and molecular facets of keratinocyte reepithelization during wound healing. Exp Cell Res. 2005;304(1):274–86.
Matus CE, et al. Activation of the human keratinocyte B1 bradykinin receptor induces expression and secretion of metalloproteases 2 and 9 by transactivation of epidermal growth factor receptor. Exp Dermatol. 2016;25(9):694–700.
Yousuf Y, Amini-Nik S. The role of myeloid lineage cells on skin healing and skin regeneration. J Tissue Sci Eng. 2017;8(2):1000202.
Gill SE, Parks WC. Metalloproteinases and their inhibitors: regulators of wound healing. Int J Biochem Cell Biol. 2008;40(6–7):1334–47.
Robitaille AM, et al. Quantitative phosphoproteomics reveal mTORC1 activates de novo pyrimidine synthesis. Science. 2013;80:1320–3.
Zhou B-BS, Zhang H, Damelin M, Geles KG, Grindley JC, Dirks PB. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat Rev Drug Discov. 2009;8(10):806–23.
Nguyen T-L, Durán RV. Glutamine metabolism in cancer therapy. Cancer Drug Resist. 2018;1(3):126–38.
Wise DR, Thompson CB. Glutamine addiction: a new therapeutic target in cancer. Trends Biochem Sci. 2010;35(8):427–33.
G. F. Weber, 2015 “Drugs that Suppress Proliferation,” in Molecular Therapies of Cancer, Springer, pp. 113–162.
Russell JO, Monga SP. Wnt/β-catenin signaling in liver development, homeostasis, and pathobiology. Annu Rev Pathol Mech Dis. 2018;13:351–78.
Spear BT, Jin L, Ramasamy S, Dobierzewska A. Transcriptional control in the mammalian liver: liver development, perinatal repression, and zonal gene regulation. Cell Mol Life Sci C. 2006;63(24):2922–38.
Wild SL, Elghajiji A, Grimaldos Rodriguez C, Weston SD, Burke ZD, Tosh D. The canonical wnt pathway as a key regulator in liver development, differentiation and homeostatic renewal. Genes (Basel). 2020;11(10):1163.
Rehman AU, et al. Computational approaches for the design of modulators targeting protein-protein interactions. Expert Opin Drug Discov. 2023. https://doi.org/10.1080/17460441.2023.2171396.
Molla MHR, et al. Integrative ligand-based pharmacophore modeling, virtual screening, and molecular docking simulation approaches identified potential lead compounds against pancreatic cancer by targeting FAK1. Pharmaceuticals. 2023;16(1):120.
H. Shu-Hsien, Y. U. Chih-Wen, and C. H. Lin, 2005 “Hydrogen peroxide functions as a stress signal in plants,” Bot. Bull. Acad. Sin. 46
Kinra M, Joseph A, Nampoothiri M, Arora D, Mudgal J. Inhibition of NLRP3-inflammasome mediated IL-1β release by phenylpropanoic acid derivatives: In-silico and in-vitro approach. Eur J Pharm Sci. 2021;157: 105637.
Dawood KM, Farghaly TA. Thiadiazole inhibitors: a patent review. Expert Opin Ther Pat. 2017;27(4):477–505.
Paoli P, Giannoni E, Chiarugi P. 2013 “Anoikis molecular pathways and its role in cancer progression.” Biochim Biophys Acta BBA-Mol Cell Res. 2013;1833(12):3481–98.
Iovino L, Tremblay ME, Civiero L. Glutamate-induced excitotoxicity in Parkinson’s disease: the role of glial cells. J Pharmacol Sci. 2020;144(3):151–64.
Acknowledgements
NA.
Funding
No funding.
Author information
Authors and Affiliations
Contributions
Conceptualization: AE, TES; Formal analysis; AN, AIY; Investigation: MY, MAEA; Project administration: TES, AE, AN Software: AE, AEN TES; Validation TES, AE, AIY, MY; Visualization: MAEA, AEN; Writing—original draft: AE, TES Writing—review and editing: AE; all authors have read and agreed to the published version of the manuscript. The authors declare that all data were generated in-house and that no paper mill was used.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Ethical approval
All experimental procedures used are carried out following Alexandria University's animal care guidelines and the National Science Council's Guide for the Care and Use of Laboratory Animals.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Elmetwalli, A., Nageh, A., Youssef, A.I. et al. Ammonia scavenger and glutamine synthetase inhibitors cocktail in targeting mTOR/β-catenin and MMP-14 for nitrogen homeostasis and liver cancer. Med Oncol 41, 38 (2024). https://doi.org/10.1007/s12032-023-02250-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12032-023-02250-z