Abstract
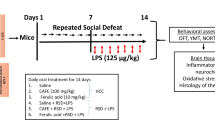
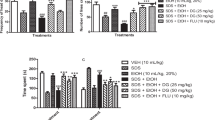
Psychosocial stress has been widely reported to contribute to psychiatric disturbances. Perturbations in the enzymes of GABAergic and cholinergic systems have been implicated as precursors in different stress-related neuropsychiatric diseases. Targeting glutamic acid decarboxylase-67 kDa (GAD67) and acetylcholinesterase (AChE) via oxidative, nitrergic, and neuroinflammatory mechanisms have been recognized as prospective strategies for the prevention of psychosocial stress-induced behavioral impairments. Naringin, a neuro-active flavonoid compound isolated from citrus fruits, has been shown to produce memory-enhancing, antiepileptic, antidepressant, and anti-inflammatory activities similarly to ginseng, a very potent adaptogen. In this communication, we assessed the effect of naringin on social-defeat stress (SDS)-induced behavioral, GABAergic, cholinergic, oxidative, nitrergic, and neuroinflammatory changes in mice using the resident–intruder paradigm. The intruder male mice were culled into six groups. Groups 1 and 2 (normal- and SDS-controls) received sterile saline, groups 3–5 were given naringin (25-100 mg/kg, i.p.) whereas group 6 had ginseng (50 mg/kg, i.p.) daily for 14 days, but followed by 10 min SDS (physical and psychological) exposure to groups 2–6 with aggressor–resident mice. Behavioral effects using Y-maze, elevated-plus maze, sociability, and tail-suspension tests were assessed on day 14. GAD67, AChE enzymes, and biomarkers of oxidative, nitrergic, and neuroinflammatory changes were assayed in the striatum, prefrontal cortex, and hippocampus. Naringin and ginseng reversed all SDS-induced behavioral impairments. Naringin increased the levels of GAD67 and decreased AChE activities in the striatum, prefrontal cortex, and hippocampus. Furthermore, naringin reduced pro-inflammatory cytokines (TNF-α, IL-6), malondialdehyde, nitrite concentrations, and increased glutathione levels in a region-dependent manner. Our study suggests that naringin attenuated SDS-induced behavioral endophenotypes of neuropsychiatric disease through increased GAD67 synthesis, inhibition of AChE activity, oxidative, nitrergic stress, and neuroinflammatory processes in stress-sensitive brain regions.









Similar content being viewed by others
References
Amsterdam J, Nutt D, Brink W (2013) Generic legislation of new psychoactive drugs. J Psychopharmacol 27:317–324
Aoyama K, Suh SW, Hamby AM, Liu J, Chan WJ, Chen Y, Swanson RA (2006) Neuronal glutathione deficiency and age-dependent neurodegeneration in the EAAC1 deficient mouse. Nat Neurosci 9:119–126
Arnarsson A, Nygren J, Nyholm M, Torsheim T, Augustine L, Bjereld Y, Markkanen I, Schnohr CW, Rasmussen M, Nielsen L, Bendtsen P (2019) Cyberbullying and traditional bullying among Nordic adolescents and their impact on life satisfaction. Scand J Public Health 48:502–510
Aroui S, Aouey B, Chtourou Y, Meunier A, Fetoui H, Kenani A (2016) Naringin suppresses cell metastasis and the expression of matrix metalloproteinases (MMP-2 and MM-9) via inhibition of ERK-P38-JNK signaling pathways in human glioblastoma. Chem Biol Interact 244:195–2013
Behrens MM, Ali SS, Laura LD (2008) Interleukin-6 mediates the increase in NADPHoxidase in the ketamine model of schizophrenia. J Neurosci 28:13957–13966
Ben-Azu B, Emokpae O, Ajayi AM, Jarikre TA, Orhode V, Aderibigbe AO, Umukoro S, Iwalewa EO (2020) Repeated psychosocial stress causes glutamic acid decarboxylase isoform- 67, oxidative-Nox-2 changes and neuroinflammation in mice: prevention by treatment with a neuroactive flavonoid, morin. Brain Res 1744:146917
Ben-Azu B, Aderibigbe AO, Ajayi AM, Eneni AO, Omogbiya IA, Owoeye EO, Umukoro S, Iwalewa EO (2019a) Morin decreases cortical pyramidal neuron degeneration via inhibition of neuroinflammation in mouse model of schizophrenia. Int Immunopharmacol 70:338–335
Ben-Azu B, Nwoke EE, Aderibigbe AO, OmogbiyaIA AAM, Olonode ET, Umukoro S, Iwalewa EO (2019b) Possible neuroprotective mechanisms of action involved in the neurobehavioral property of naringin in mice. Biomed Pharmacother 109:536–546
Ben-Azu B, Aderibigbe AO, Omogbiya IA, Ajayi AM, Owoeye O, Olonode ET, Iwalewa EO (2018) Probable mechanisms involved in the antipsychotic-like activity of morin in mice. Biomed Pharmacother 105:1079–1090
Bisht K, Sharma K, Lecours C, Sanchez MG, El-Hajj H, Milior G, Olmos-Alonso A, Gomez-Nicola D, Luheshi G, Vallières L, Branchi I, Maggi L, Livimatola C, Butovsky O, Tremblay ME (2016) Dark microglia: a new phenotype predominantly associated with pathological states. Glia 64:826–839
Björkqvist K (2001) Social defeat as a stressor in humans. Physiol Behav 73:435–442
Block ML, Zecca L, Hong JS (2007) Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci 8:57–69
Bouvier E, Brouillard F, Molet J, Claverie D, Cabungcal JH, Cresto N, Doligez N, Rivat C, Do KQ, Bernard C, Benoliel JJ, Becker C (2017) Nrf2-dependent persistent oxidative stress results in stress-induced vulnerability to depression. Mol Psychiatry 22:1701–1713
Bowers G, Cullinan WE, Herman JP (1998) Region-specific regulation of glutamic acid decarboxylase (GAD) mRNA expression in central stress circuits. J Neurosci 18:5938–5947
Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry GRL, Bowen MAS (2006) Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta 1762:447–452
Charlson F, Ommeren M, Laxman A, Cornett J, Whiteford H, Saxena H (2019) New WHO prevalence estimates of mental disorders in conflict settings: a systematic review and meta-analysis. Lancet 394:240–248
Chtourou Y, Fetoui H, Gdoura R (2014) Protective effects of naringenin on iron-overload induced cerebral cortex neurotoxicity correlated with oxidative stress. Biol Trace Elem Res 158:376–383
Cryan JF, Holmes A (2005) The ascent of mouse: advances in modelling human depression and anxiety. Nat Rev Drug Discov 4:775–790
Czeh B, Simon M, van der Hart MG (2005) Chronic stress decreases the number of parvalbumin-immunoreactive interneurons in the hippocampus: prevention by treatment with a substance P receptor (NK1) antagonist. Neuropsychopharmacology 30:67–79
Dricks S (2016) Effects of neonatal stress on gamma oscillations in hippocampus. Sci Rep 6:29007
Ellman GL, Courtney KD, Andres V, Jr Feather-Stone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Fernandez SP, Michael N, Yow TT, Chu C, Graham AR, Johnston AR, Hanrahan JR, Chebib M (2009) The flavonoid glycosides, Myricitrin, Gossypin and naringin exert anxiolytic action in mice. Neurochem Res 34:1867–1875
Ferrari AJ, Charlson FJ, Norman RE, Patten SB, Freedman G, Murray CJ, Vos T, Whiteford HA (2013) Burden of depressive disorders by country, sex, age, and year: findings from the global burden of disease study. PLoS Med 10:e1001547
File SE, Kenny PJ, Cheeta S (2000) The role of the dorsal hippocampal serotonergic andcholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav 66:65–72
Gaurav P, Solanki N, Atrooz F, Allam F, Salim S (2013) Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res 1539:73–86
Gold PE (2003) Acetylcholine modulation of neural systems involved in learning and memory. Neurobiol Learn Mem 80:194–210
Golechha M, Sarangal V, Bhatia J, Chaudhry U, Saluja D, Arya DS (2014) Naringin ameliorates pentylenetetrazol-induced seizures and associated oxidative stress, inflammation, and cognitive impairment in rats: possible mechanisms of neuroprotection. Epilepsy Behav 41:98–102
Gopinath K, Sudhandiran G (2015) Neuroprotective efficacy of Naringin on 3-nitropropionic acid-induced mitochondrial dysfunction through the modulation of Nrf2 signaling pathway in PC12 cells. Mol Cell Biochem 409:199–211
Gopinath K, Sudhandiran G (2012) Naringin modulates oxidative stress and inflammation in 3-nitropropionic acid-induced neurodegeneration through the activation of nuclear factor erythroid 2-related factor-2 signalling pathway. Neuroscience 227:134–143
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum protein by means of biuret reaction. J Biol Chem 177:751–766
Green LC, Tannenbaum SR, Goldman P (1981) Nitrate synthesis in the germ free and conventional rat. Science 212:56–58
Hammels C, Pishva E, De Vry J, Van den Hove DLA, Prickaerts J, Winkel RV, Selten JP (2015) Defeats stress in rodents: from behavior to molecules. Neurosci Biobehav Rev 59:111–140
Hanrahan JR, Chebib M, Johnston GA (2011) Flavonoid modulation of GABA(a) receptors. Br J Pharmacol 163:234–245
Hosseinzadeh H, Nassiri-Asl M (2017) Neuroprotective Effects of Flavonoids in Epilepsy: Clinical Aspects and Mode of Action. https://doi.org/10.1002/9783527803781.ch10
Janowsky DS, El-Yousef MK, Davis JM, Sekerke HJ (1972) A cholinergic-adrenergichypothesis of mania and depression. Lancet 2:632–635
Jing D, Ming Z, Hongkun B, Bai L, Yilong D, Chunjie X, Grace YZ, Ioline H, Matthew R, Benedetto V (2016) The role of nutrients in protecting mitochondrial function and neurotransmitter signaling: implications for the treatment of depression, PTSD, and suicidal behaviors. Crit Rev Food Sci Nutr 6:2560–2578
Jollow DJ, Michell JR, Zampaglione N, Gillete J (1974) Bromobenzene- induced liver necrosis. Protective role of glutathione an evidence for 3,4bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Jung UJ, Kim SR (2014) Effects of naringin, a flavanone glycoside in grapefruits and citrus fruits, on the nigrostriatal dopaminergic projection in the adult brain. Neural Regen Res 9:1514–1517
Karolewicz B, Maciag D, O’Dwyer G (2010) Reduced level of glutamic acid decarboxylase-67 kDa in the prefrontal cortex in major depression. Int J Neuropsychopharmacol 13:411–420
Kim DH, Jung EA, Sohng IS, Han JA, Kim TH, Han MJ (1998) Intestinal bacterial metabolism of flavonoids and its relation to some biological activities. Arch Pharm Res 21:17–23
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, LaPlant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131:391–404
Kwatra M, Ashok J, Murli M, Yogita S, Sahabuddin A, Pinaki G, Vikas K, Divya V, Razia K (2016) Naringin and sertraline ameliorate doxorubicin-induced behavioral deficits through modulation of serotonin level and mitochondrial complexes protection pathway in rat hippocampus. Neurochem Res 41:2352–2366
Lau CG, Murthy VN (2012) Activity-dependent regulation of inhibition via GAD67. J Neurosci 32:8521–8531
Lázaro-Visa S, Palomera R, Briones E, Fernández-Fuertes AA, Fernández-Rouco N (2019) Bullied adolescent’s life satisfaction: personal competencies and school climate as protective factors. Front Psychol 10:1691
Lee S, Rhee DK (2017) The effects of ginseng on stress-related depression, anxiety, and the hypothalamic-pituitary-adrenal axis. J Ginseng Res 41:589–594
Lewis DA, Cho RY, Carter CS, Eklund K, Forster S, Kelly MA, Montrose D (2008) Subunit-selective modulation of GABA type a receptor neurotransmission and cognition in schizophrenia. Am J Psychiatry 165:1585–1593
Iio W, Takagi H, Ogawa Y, Tsukahara T, Chohnan S, Toyoda A (2014) Effects of chronic social defeat stress on peripheral leptin and its hypothalamic actions. BMC Neuroscience 15 (1):72
Iio W, Tokutake Y, Matsukawa N, Tsukahara T, Chohnan S, Toyoda A (2012) Anorexic behavior and elevation of hypothalamic malonyl-CoA in socially defeated rats. Biochem. Biophy Res Com 421:301–304
Ma Q (2013) Role of Nrf2 in oxidative stress and toxicity. Annu Rev Pharmacol Toxicol 52:401–426
MacDowell KS, Garcıa-Bueno B, Madrigall JLM, Parellada M, Arango C, Mico JA (2013) Risperidone normalizes increased inflammatory parameters and restores anti-inflammatory pathways in a model of neuroinflammation. Int J Neuropsychopharmacol 16:121–135
Macedo GC, Morita GM, Domingues LP, Favoretto CA, Suchecki D, Quadros IMH (2018) Consequences of continuous social defeat stress on anxiety- and depressive-like behaviors and ethanol reward in mice. Horm Behav 97:154–161
Maki RA, Tyurin VA, Lyon RC, Hamilton RL, DeKosky ST, Kagan VE, Reynolds WF (2009) Aberrant expression of myeloperoxidase in astrocytes promotes phospholipid oxidation and memory defcits in a mouse model of Alzheimer disease. J Biol Chem 284:3158–3169
Makinson R, Lundgren KH, Seroogy KB, Herman JP (2015) Chronic social subordination stress modulates glutamic acid decarboxylase (GAD) 67 mRNA expression in central stress circuits. Physiol Behav 1(146):7–15
Maratha SR, Mahadevan N (2012) Memory enhancing activity of naringin in unstressed and stressed mice. Possible cholinergic and nitriergic modulation. Neurochem Res 37:2206–2212
McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, Godbout JP (2016) Neuroinflammatory dynamics underlie memory impairments after repeated social defeat. J Neurosci 36:2590–2604
Meerson A, Cacheaux L, Goosens KA, Sapolsky RM, Soreq H, Kaufer D (2010) Changes in brain MicroRNAs contribute to cholinergic stress reactions. J Mol Neurosci 40:47–55
Miczek A, Yap J, Covington H (2008) Social stress, therapeutics and drug abuse: preclinical models of escalated and depressed intake. Pharmacol Ther 120:102–128
Miller AH, Raison CL (2015) The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat Rev Immunol 16:22–34
Mineur YS, Obayemi A, Wigestrand MB, Fote GM, Calarco CA, Li AM, Picciotto RM (2013) Cholinergic signaling in the hippocampus regulates social stress resilience and anxiety- and depression-like behavior. Proc Natl Acad Sci U S A 110:3573–3578
Miranda R, Granado XO, Amutio A, Ortúzar H (2018) Adolescent bullying victimization and life satisfaction: can family and school adult support figures mitigate this effect? Revista de Psicodidáctica. https://doi.org/10.1016/j.psicoe.2018.07.001
Monte AS, de Souza GC, Mclntyre RS, Joanna KS, dos Santos JV, Rafaela CC, Bruna MM, de Lucena RDF, Vasconcelos SMW, de Sousa FCF, Carvalho AF, Macêdo DS (2013) Prevention and reversal of ketamine-induced schizophrenia related behavior by minocycline in mice: possible involvement of antioxidant and nitrergic pathway. J Psychopharmacol 27:1032–1043
Nishi MH, Hayashi N, Sasagawa T (2014) Effects of early life adverse experiences on the brain: implications from maternal separation models in rodents. Front Neurosci 8:166
Okhawa H, Ohishi N, Yagi E (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Panossian AG (2013) Adaptogens in mental and behavioral disorders. Psychiatric Clin North Am 36:49–64
Prakash A, Shur B, Kumar A (2013) Naringin protects memory impairment and mitochondrial oxidative damage against aluminum-induced neurotoxicity in rats. Int J Neurosci 123:636–645
Punithavathi VR, Anuthama R, Prince PS (2008) Combined treatment with naringin and vitamin C ameliorates streptozotocin-induced diabetes in male Wistar rats. J Appl Toxicol 28:806–813
Reader BF, Jarrett BL, McKim DB, Wohleb ES, Godbout JP, Sheridan JF (2015) Peripheral and central effects of repeated social defeat stress: monocyte trafficking, microglial activation and anxiety. Neuroscience 289:429–442
Rygula R, Abumaria N, Flugge G, Fuchs E, Ruther E, Havemann-Reinecke U (2005) Anhedonia and motivational deficits in rats: impact of chronic social stress. Behav Brain Res 162:127–134
Savah S, Montserrat C, Casas F, Adams S, Tiliouine H, Benninger S, Jackson K (2018) Children’s experiences of bullying victimization and the influence on their subjective well-being: a multinational comparison. Child Dev 90:414–431
Schiavone S, Jaquet V, Sorce S, Dubois-Dauphin M, Hultqvist M, Bäckdah L, Holmdah R, Colaianna M, Cuomo V, Trabace L, Krause KH (2012) NADPH oxidase elevations in pyramidal neurons drive psychosocial stress-induced neuropathology. Transl Psychiatry 2:e111
Shu-Huei T, Shoei-Yn L, Jen-Kun L (1999) Suppression of nitric oxide synthase and the down-regulation of the activation of NFκB in macrophages by resveratrol. Br J Pharmacol 126:673–680
Serra-Negra JM, Paiva SM, Bendo CB, Fulgêncio LB, Lage CF, Corrêa-Faria F, Pordeus IA (2015) Verbal school bullying and life satisfaction among Brazilian adolescents: profiles of the aggressor and the victim. Compr Psychiatry 57:132–139
Stein DJ, Vasconcelos MF, Albrechet-Souza L, Ceresér KMM, de Almeida RMM (2017) Microglial over-activation by social defeat stress contributes to anxiety- and depressive-like behaviors. Front Behav Neurosci 11:207
Steru L, Chermat R, Thierry B, Simon P (1985) The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology 85:367–370
Suzuki H, Ohgidani M, Kuwano N, Chrétien F, Lorin de la Grandmaison J, Onaya M, Tominaga I, Setoyama D, Kang D, Mimura M, Kanba S, Kato TA (2019) Suicide and microglia: recent findings and future perspectives based on human studies. Front Cell Neurosci 13:31
Tang J, Yu W, Chen S, Gao Z, Xiao B (2018) Microglia polarization and endoplasmic reticulum stress in chronic social defeat stress induced depression mouse. Neurochem Res 43:985–994
Tonelli C, Iok In Christine C, Tuveson AD (2018) Transcriptional regulation by Nrf2. Antioxid Redox Signal 29:1727–1745
Tucci T, Cheeta S, Seth P, File SE (2003) Corticotropin releasing factor antagonist, alpha-helical CRF(9-41), reverses nicotine-induced conditioned, but not unconditioned,anxiety. Psychopharmacology 167:251–256
Ulrich-Lai YM, Herman JP (2009) Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci 10:397–409
Umukoro S, Kalejaye HA, Ben-Azu B, Ajayi AM (2018) Naringenin attenuates behavioral derangements induced by social defeat stress in mice via inhibition of acetylcholinesterase activity, oxidative stress and release of pro-inflammatory cytokines. Biomed Pharmacother 105:714–723
Venzala E, García-García AL, Elizalde N, Delagrange P, Tordera RM (2012) Chronic social defeat stress model: behavioral features, antidepressant action, and interaction with biological risk factors. Psychopharmacology 224:313–325
Wan Ismail WS, Nik Jaafar NR, Sidi H, Midin M, Shah SA (2014) Why do young adolescents bully? Experience in Malaysian schools. Compr Psychiatry 55:S114–S120
Wang DM, Yang YJ, Zhang L, Zhang X, Guan FF, Zhang LF (2013) Naringin enhances CaMKII activity and improves long-term memory in a mouse model of Alzheimer’s disease. Int J Mol Sci 14:5576–5586
Wong KC, Pang YW, Wang XL, Mok SK, Lai WP, Chow HK, Leung PC, Wong MS (2013) Drynaria fortunei-derived total flavonoid fraction and isolated compounds exert oestrogen like protective effects in bone. Bri J Nutr 110:475–485
Zhang Y, Wang Q, Chen H, Liu X, Lv K, Wang T, Wang Y, Ji G, Cao H, Kan G, Li Y, Qu L (2018) Involvement of cholinergic dysfunction and oxidative damage in the effects of simulated weightlessness on learning and memory in rats. Biomed Res Int 2018:2547532
Acknowledgements
The Authors are grateful to the technical staff of the Department of Pharmacology and Therapeutics, Faculty of Basic Medical Sciences, University of Ibadan for their technical assistance during the study.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All experiments were approved and performed under the guidelines of the University of Ibadan’s Animals Ethic Committee (ACUREC/App/06/2018/18) and the National Institutes of Health Guide for Care and Use of Laboratory Animals (Publication number: 85-23, revised 1985).
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Oladapo, O.M., Ben-Azu, B., Ajayi, A.M. et al. Naringin Confers Protection against Psychosocial Defeat Stress-Induced Neurobehavioral Deficits in Mice: Involvement of Glutamic Acid Decarboxylase Isoform-67, Oxido-Nitrergic Stress, and Neuroinflammatory Mechanisms. J Mol Neurosci 71, 431–445 (2021). https://doi.org/10.1007/s12031-020-01664-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-020-01664-y