Abstract
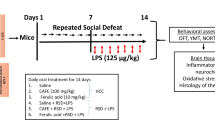
Social defeat stress (SDS) due to changes in biochemical functions has been implicated in the pathogenesis of affective and cognitive disorders. Employing pharmacological approach with adaptogens in the management and treatment of psychosocial stress is increasingly receiving scientific attention. In this study, we investigated the neuroprotective effect of rutin, a bioflavonoid with neuroprotective and anti-inflammatory functions on neurobehavioral and neuro-biochemical changes in mice exposed to SDS. Groups of mice named the intruder mice received normal saline (10 mL/kg), rutin (5, 10, and 20 mg/kg, i.p.), and ginseng (50 mg/kg, i.p.) daily for 14 days, and then followed by 10 min daily SDS (physical/psychological) exposures to aggressor mice from days 7-14. Investigations consisting of neurobehavioral (locomotion, memory, anxiety, and depression) phenotypes, neuro-biochemical (oxidative, nitrergic, cholinergic, and pro-inflammatory cytokines) levels in discrete brain regions, and hypothalamic–pituitary–adrenal (HPA) axis consisting adrenal weight, corticosterone, and glucose concentrations were assessed. Rutin restored the neurobehavioral deficits and reduced the activity of acetylcholinesterase in the brains. Adrenal hypertrophy, increased serum glucose and corticosterone levels were significantly attenuated by rutin. SDS-induced release of tumor necrosis factor-alpha and interleukin-6 in the striatum, prefrontal cortex, and hippocampus were also suppressed by rutin in a brain-region-dependent manner. Moreover, SDS-induced oxidative stress characterized by low antioxidants (glutathione, superoxide-dismutase, catalase) and lipid peroxidation and nitrergic stress were reversed by rutin in discrete brain regions. Collectively, our data suggest that rutin possesses an adoptogenic potential in mice exposed to SDS via normalization of HPA, oxidative/nitrergic, and neuroinflammatory inhibitions. Thus, may be adopted in the management of neuropsychiatric syndrome due to psychosocial stress.








Similar content being viewed by others
Data Availability
Data is available if reasonably requested to the authors.
Code Availability
Not applicable.
References
Abe R, Okada S, Nakayama R (2019) Social defeat stress causes selective attenuation of neuronal activity in the ventromedial prefrontal cortex. Sci Rep 9:9447
Adams SV, Winterer J, Muller W (2004) Muscarinic signaling is required for spike-pairing induction of long-term potentiation at rat Schaffer collateral-CA1 synapses. Hippocampus 14:413–416
Adebayo OG, Ben-Azu B, Ajayi AM, Wopara I, Aduema W, Kolawole TA, Umoren EB, Onyeleonu I, Ebo OT, Ajibo DN, Akpotu AE (2021) Gingko biloba abrogate lead-induced neurodegeneration in mice hippocampus: involvement of NF-κB expression, myeloperoxidase activity and pro-inflammatory mediators. Biol Trace Elem Res 9:1–4
Aderibigbe AO, Iwalewa EO, Adesina SK, Agboola OI (2010) Studies of behavioural and neural mechanisms of Aridanin isolated from tetrapleura tetraptera in mice. Int J Pharmacol 6:480–486
Anjomshoa M, Boroujeni SN, Ghasemi S, Lorigooini Z, Amiri A, Balali-Dehkordi S, Amini-Khoei H (2020) Rutin via Increase in the CA3 Diameter of the hippocampus exerted antidepressant-like effect in mouse model of maternal separation stress: possible involvement of NMDA receptors. Behav Neurol 2020:4813616
Ben-Azu B, Adebayo OG, Jarikre TA, Oyovwi MO, Edje KE, Omogbiya IA, Eduviere AT, Moke EG, Chijioke BS, Odili OS, Omondiabge OP, Oyovbaire A, Esuku DT, Ozah EO, Japhet K (2022) Taurine, an essential β-amino acid insulates against ketamine-induced experimental psychosis by enhancement of cholinergic neurotransmission, inhibition of oxidative/nitrergic imbalances, and suppression of COX-2/iNOS immunoreactions in mice. Metab Brain Dis. https://doi.org/10.1007/s11011-022-01075-5
Ben-Azu B, Emokpae O, Ajayi AM, Jarikre TA, Orhode V, Aderibigbe AO, Umukoro S, Iwalewa EO (2020) Repeated psychosocial stress causes glutamic acid decarboxylase isoform-67, oxidative-Nox-2 changes and neuroinflammation in mice: prevention by treatment with a neuroactive flavonoid, morin. Brain Res 1744:146917
Ben-Azu B, Aderibigbe AO, Omogbiya IA, Ajayi AM, Owoeye O, Olonode ET, Iwalewa EO (2018a) Probable mechanisms involved in the antipsychotic-like activity of morin in mice. Biomed Pharmacother 105:1079–1090
Ben-Azu B, Aderibigbe AO, Ajayi AM, Eneni AO, Umukoro S, Iwalewa EO (2018b) Involvement of GABAergic, BDNF and Nox-2 mechanisms in the preventionand reversal of ketamine-induced schizophrenia-like behavior by morin in mice. Brain Res Bull 139:292–206
Ben-Azu B, Omogbiya IA, Aderibigbe AO, Umukoro S, Ajayi AM, Iwalewa EO (2018c) Doxycycline prevents and reverses schizophrenic-like behaviors induced by ketamine in mice via modulation of oxidative, nitrergic and cholinergic pathways. Brain Res Bull 139:114–124
Ben-Azu B, Aderibigbe AO, Ajayi AM, Iwalewa EO (2016) Neuroprotective effects of the ethanol stem bark extracts of Terminalia ivorensis in ketamine-induced schizophrenia-like behaviors and oxidative damage in mice. Pharm Biol 54(12):2871–2879
Bonfiglio JJ, Inda C, Refojo D, Holsboer F, Arzt E, Silberstein S (2011) The corticotropin-releasing hormone network and the hypothalamic-pituitary-adrenal axis: molecular and cellular mechanisms involved. Neuroendocrinology 94:12–20
Bourin M, Hascoet M (2003) The mouse light/dark box test. Eur J Pharmacol 463:55–65
Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA (2006) Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochem Biophys Acta 1762:447–452
Charlson F, Ommeren M, Flaxman A, Cornett J, Whiteford H, Saxena S (2019) New WHO prevalence estimates of mental disorders in conflict settings: a systematic review and meta-analysis. Lancet 394:240–48
Chen P, Fan Y, Li Y, Sun Z, Bissette G, Zhu MY (2012) Chronic social defeat up-regulates expression of norepinephrine transporter in rat brains. Neurochem Int 60(1):9–20
Dunn AJ, Swiergiel AH (2008) The role of corticotropin-releasing factor and noradrenaline in stress-related responses, and the inter-relationships between the two systems. Eur J Pharmacol 583:186–193
Eduviere AT, Umukoro S, Adeoluwa OA, Omogbiya IA, Aluko OM (2016) Possible mechanisms involved in attenuation of lipopolysaccharide-induced memory deficits by methyl jasmonate in mice. Neurochem Res 41(12):3239–3249
Eduviere AT, Umukoro S, Aderibigbe AO, Ajayi AM, Adewole FA (2015) Methyl jasmonate enhances memory performance through inhibition of oxidative stress and acetylcholinesterase activity in mice. Life Sci 132:20–26
Elizabeth A, Adegbuyi A, Olusegun A, Benneth BA, Anthony E, Abayomi A, Solomon U (2020) Morin hydrate attenuates chronic stress-induced memory impairment and degeneration of hippocampal subfields in mice: the role of oxidative, nitrergic and neuroinflammatory pathways. Metab Brain Dis 35:1145–1156
Ellman GL, Courtney KD, Andres V Jr, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7(2):88–95
Emokpae O, Ben-Azu B, Ajayi AM, Umukoro S (2020) D-Ribose-L-cysteine attenuates lipopolysaccharide-induced memory deficits through inhibition of oxidative stress, release of proinflammatory cytokines, and nuclear factor-kappa B expression in mice. Naunyn-Schmied Arch Pharm 393:909–925
Fanous S, Hammer RP Jr, Nikulina EM (2010) Short-and long-term effects of intermittent social defeat stress on brain-derived neurotrophic factor expression in mesocorticolimbic brain regions. Neuroscience 167(3):598–607
Gaurav P, Solanki N, Atrooz F, Allam F, Salim S (2013) Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res 1539:73–86
Gornall AG, Bardawill CJ, David MM (1949) Determination of serum protein by means of Biuret Reaction. J Biol Chem 177:751–766
Green LC, Tannenbaum SR, Goldman P (1981) Nitrate synthesis in the germ free and conventional rat. Science 212:56–58
Hasegawa S, Miyake Y, Yoshimi A, Mouri A, Hida H, Yamada K, Ozaki N, Nabeshima T, Noda Y (2018) Dysfunction of serotonergic and dopaminergic neuronal systems in the antidepressant-resistant impairment of social behaviors induced by social defeat stress exposure as juveniles. Int J Neuropsychopharmacol 21(9):837–846
Hosseinzadeh H, Nassiri-Asl M (2014) Review of the protective effects of rutin on the metabolic function as an important dietary flavonoid. J Endocrinol Invest 37:783–788
Hughes MM, Carballedo A, McLoughlin DM, Amico F, Harkin A, Frodl T, Connor TJ (2012) Tryptophan depletion in depressed patients occurs independent of kynurenine pathway activation. Brain Behav Immun 26(6):979–987
Johnson JD, David FB, Adam CK, Devanshi MM (2019) Neuroendocrine regulation of brain cytokines after psychological stress. J Endocr Soc 3(7):1302–1320
Jollow DJ, Michell JR, Zampaglione N, Gillete J (1974) Bromobenzene- induced liver necrosis. Protective role of glutathione an evidence for 3,4 bromobenzene oxide as the hepatotoxic metabolite. Pharmacology 11:151–169
Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ (2007) Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell 131:391–404
Lee S, Rhee DK (2017) The effects of ginseng on stress-related depression, anxiety, and the hypothalamic-pituitary-adrenal axis. J Ginseng Res 41:589–594
Machado DG, Bettio LE, Cunha MP, Santos AR, Pizzolatti MG, Brighente IM, Rodrigues AL (2008) Antidepressant-like effect of rutin isolated from the ethanolic extract from Schinus molle L. in mice: evidence for the involvement of the serotonergic and noradrenergic systems. Eur J Pharmacol 587(1–3):163–8
Mallei A, Ieraci A, Popoli M (2019) Chronic social defeat stress differentially regulates the expression of BDNF transcripts and epigenetic modifying enzymes in susceptible and resilient mice. World J Biol Psychiatry. 20(7):555–566
McAllister BB, Pochakom A, Fu S, Dyck RH (2020) Effects of social defeat stress and fluoxetine treatment on neurogenesis and behavior in mice that lack zinc transporter 3 (ZnT3) and vesicular zinc. Hippocampus 30:623–637
McEwen BS, Gray JD, Nasca C (2015) Recognizing resilience: learning from the effects of stress on the brain. Neurobiol Stress 1:1–11
McKim DB, Niraula A, Tarr AJ, Wohleb ES, Sheridan JF, Godbout JP (2016) Neuroinflammatory dynamics underlie memory impairments after repeated social defeat. J Neurosci 36:2590–2604
McLaughlin KA, Green JG, Gruber MJ, Sampson NA, Zaslavsky AM, Kessler RC (2010) Childhood adversities and adult psychopathology in the National Comorbidity Survey Replication (NCS-R) III: associations with functional impairment related to DSM-IV disorders. Psychol Med 40:847–859
Miczek KA, Hussain S, Faccidomo S (1998) Alcohol-heightened aggression in mice: attenuation by 5-HT 1A receptor agonists. Psychopharmacology 139(1–2):160–168
Misra HP, Fridovich I (1972) The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem 247:3170–3175
Montagud-Romero S, Nuñez C, Blanco-Gandia MC, Martínez-Laorden E, Aguilar MA, Navarro-Zaragoza J, Almela P, Milanés MV, Laorden ML, Miñarro J, Rodríguez-Arias M (2017) Repeated social defeat and the rewarding effects of cocaine in adult and adolescent mice: dopamine transcription factors, proBDNF signaling pathways, and the TrkB receptor in the mesolimbic system. Psychopharmacology 234:2063–2075
Moravcová S, Červená K, Míková H, Pačesová D, Pallag G, Novotný J, Bendová Z (2021) Social defeat stress affects resident’s clock gene and bdnf expression in the brain. Stress 24(2):206–212
Morgan C, Gayer-Anderson C, Beards S, Hubbard K, Mondelli V, Di Forti M, Murray RM, Pariante C, Dazzan P, Craig TJ, Reininghaus U, Fisher HL (2020) Threat, hostility and violence in childhood and later psychotic disorder: population-based case-control study. Br J Psychiatry 217(4):575–582
Mouri A, Ukai M, Uchida M, Hasegawa S, Taniguchi M, Ito T, Hida H, Yoshimi A, Yamada K, Kunimoto S, Ozaki N, Nabeshima T, Noda Y (2018) Juvenile social defeat stress exposure persistently impairs social behaviors and neurogenesis. Neuropharmacology 133:23–37
Muscat R, Papp M, Willner P (1992) Reversal of stress, induced anhedonia by the atypical antidepressants, fluoxetine and maprotiline. Psychopharmacol Berl 109:433–438
Niraula A, Witcher KG, Sheridan JF, Godbout JP (2019) Interleukin-6 induced by social stress promotes a unique transcriptional signature in the monocytes that facilitate anxiety. Biol Psychiatry 85:679–689
Niraula A, Wang Y, Godbout JP, Sheridan JF (2018) Corticosterone production during repeated social defeat causes monocyte mobilization from the bone marrow, glucocorticoid resistance, and neurovascular adhesion molecule expression. J Neurosci 38:2328–2340
Nishi M, Horii-Hayashi N, Sasagawa T (2014) Effects of early life adverse experiences on the brain: implications from maternal separation models in rodents. Front Neurosci 8:166
Okhawa H, Ohishi N, Yagi E (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–435
Okoh L, Ajayi AM, Ben-Azu B, Akinluyi ET, Emokpae O, Umukoro S (2020a) d-Ribose-l-cysteine exhibits adaptogenic-like activity through inhibition of oxido-infammatory responses and increased neuronal caspase-3 activity in mice exposed to unpredictable chronic mild stress. Mol Biol Rep. https://doi.org/10.1007/s11033-020-05845-1
Okoh L, Ajayi AM, Ben-Azu B, Akinluyi ET, Emokpae O, Umukoro S (2020b) D-Ribose-L-cysteine exhibits adaptogenic-like activity through inhibition of oxido-inflammatory responses and increased neuronal caspase-3 activity in mice exposed to unpredictable chronic mild stress. Mol Biol Rep 47:7709–7722
Okubo Eneni AE, Ben-Azu B, Ajayi AM, Aderibigbe AO (2020) Diosmin attenuates schizophrenia-like behavior, oxidative stress, and acetylcholinesterase activity in mice. Drug Metab Pers Ther 35(4)
Oladapo OM, Ben-Azu B, Ajayi AM, Emokpae O, Eneni AO, Omogbiya IA, Iwalewa EO (2021) Naringin confers protection against psychosocial defeat stress-induced neurobehavioral deficits in mice: involvement of glutamic acid decarboxylase isoform-67, oxido-nitrergic stress, and neuroinflammatory mechanisms. J Mol Neurosci 71:431–445
Olonode ET, Aderibigbe AO, Adeoluwa OA, Eduviere AT, Ben-Azu B (2019) Morin hydrate mitigates rapid eye movement sleep deprivation-induced neurobehavioural impairments and loss of viable neurons in the hippocampus of mice. Behav Brain Res 356:518–525
Olugbemide AS, Ben-Azu B, Bakre AG, Ajayi AM, Femi-Akinlosotu O, Umukoro S (2021) Naringenin improves depressive-and anxiety-like behaviors in mice exposed to repeated hypoxic stress through modulation of oxido-inflammatory mediators and NF-kB/BDNF expressions. Brain Res Bull 169:214–227
Oshodi TO, Ben-Azu B, Ishola IO, Ajayi AM, Emokpae O, Umukoro S (2021) Molecular mechanisms involved in the prevention and reversal of ketamine-induced schizophrenia-like behavior by rutin: the role of glutamic acid decarboxylase isoform-67, cholinergic, Nox-2-oxidative stress pathways in mice. Mol Biol Rep 48:2335–2350
Panossian A, Seo E, Efferth T (2018) Novel molecular mecha- nisms for the adaptogenic effects of herbal extracts on isolated brain cells using systems biology. Phytomedicine 50:257–284
Patel H, McIntire J, Ryan S, Dunah A, Loring R (2017) Anti-inflammatory effects of astroglial α7 nicotinic acetylcholine receptors are mediated by inhibition of the NF-κB pathway and activation of the Nrf2 pathway. J Neuroinflammation 14:192
Patki G, Naimesh S, Fatin A, Farida A, Samina S (2013) Depression, anxiety-like behavior and memory impairment are associated with increased oxidative stress and inflammation in a rat model of social stress. Brain Res 1539:73–86
Ramalingayya GV, Nampoothiri M, Nayak PG, Kishore A, Shenoy RR, Rao CM, Nandakumar K (2016) Naringin and rutin alleviates episodic memory deficits in two differentially challenged object recognition tasks. Pharmacogn Mag 12(Suppl 1):S63
Santoft F, Hedman-Lagerlöf E, Salomonsson S, Lindsäter E, Ljótsson B, Kecklund G, Lekander M, Andreasson A (2020) Inflammatory cytokines in patients with common mental disorders treated with cognitive behavior therapy. Brain Behav Immun-Health 3:100045
Sharma DK (2018) Physiology of stress and its managements. HSOA J Med: Stud Res 1:1–5
Singh S, Husain T, Kushwaha BK, Suhel M, Fatima A, Mishra V, Singh SK, Bhatt JA, Rai M, Prasad SM, Dubey NK (2021) Regulation of ascorbate-glutathione cycle by exogenous nitric oxide and hydrogen peroxide in soybean roots under arsenate stress. J Hazard Mater 409:123686
Sinha KA (1971) Colorimetric assay of catalase. Anal Biochem 47:389–394
Slee EA, Adrain C, Martin SJ (2001) Executioner caspase-3, -6, and -7 perform distinct, non-redundant roles during the demolition phase of apoptosis. J Biol Chem 276:7320–7326
Stranahan AM, Mattson MP (2012) Recruiting adaptive cellular stress responses for successful brain ageing. Nat Rev Neurosci 13:209–216
Tonnies E, Trushina E (2017) Oxidative stress, synaptic dysfunction, and Alzheimer’s disease. J Alzh Dis 57:1105–1121
Tsigos C, Kyrou I, Kassi E, Chrousos GP (2016) Stress, endocrine physiology and pathophysiology. In: Feingold KR, Anawalt B, Boyce A et al. editors. Endotext [Internet]. South Dartmouth (MA): MDText.com, Inc.; 2000-. Available from: https://www.ncbi.nlm.nih.gov/books/NBK278995/
Ugwu PI, Ben-Azu B, Ugwu SU, Uruaka CI, Nworgu CC, Okorie PO, Okafor KO, Anachuna KK, Elendu MU, Ugwu AO, Anyaehie UB, Nwankwo AA, Osim EE (2022) Preventive putative mechanisms involved in the psychopathologies of mice passively coping with psychosocial defeat stress by quercetin. Brain Res Bull 183:127–141
Umukoro S, Adeola KH, Ben-Azu B, Ajayi AM (2018) Naringenin attenuates behavioral derangements induced by social defeat stress in mice via inhibition of acetyl-cholinesterase activity, oxidative stress and release of pro- inflammatory cytokines. Biomed Pharmacother 105:714–723
Umukoro S, Aluko OM, Eduviere AT, Owoeye O (2016) Evaluation of adaptogenic-like property of methyl jasmonate in mice exposed to unpredictable chronic mild stress. Brain Res Bull 121:105–114
Van Bokhoven P, Oomen CA, Hoogendijk WJ, Smit AB, Lucassen PJ, Spijker S (2011) Reduction in hippocampal neurogenesis after social defeat is long-lasting and responsive to late antidepressant treatment. Eur J Neurosci 33:1833–1840
Venzala E, Garcia-Garcia AL, Elizarde N, Delagrange P, Tordera RM (2012) Chronic social defeat stress model: behavioural features, antidepressant action, and interaction with biological risk factors. Psychopharmacology 224:313–325
Walker BR (2007) Glucocorticoids and cardiovascular disease. Eur J Endocrinol 157:545–559
Weber MD, Godbout JP, Sheridan JF (2017) Repeated social defeat, neuroinflammation, and behavior: monocytes carry the signal. Neuropsychopharmacology 42:46–61
Wohleb ES, McKim DB, Shea DT, Powell ND, Tarr AJ, Sheridan JF, Godbout JP (2014) Re-establishment of anxiety in stress-sensitized mice is caused by monocyte trafficking from the spleen to the brain. Biol Psychiat 75:970–981
Xu H, Wang J, Zhang K, Zhao M, Ellenbroek B, Shao F, Wang W (2018) Effects of adolescent social stress and antidepressant treatment on cognitive inflexibility and Bdnf epigenetic modifications in the mPFC of adult mice. Psychoneuroendocrinol 88:92–101
Zalcman S, Green-Johnson JM, Murray L, Nance DM, Dyck D, Anisman H (1994) Cytokine-specific central monoamine alterations induced by interleukin-1, -2 and -6. Brain Res 643:40–49
Zhao D, Xu X, Pan L, Zhu W, Fu X, Guo L, Lu Q, Wang J (2017) Pharmacologic activation of cholinergic alpha7 nicotinic receptors mitigates depressive-like behavior in a mouse model of chronic stress. J Neuroinflammation 14:234
Zhou D, Kusnecov AW, Shurin MR, DePaoli M, Rabin BS (1993) Exposure to physical and psychological stressors elevates plasma interleukin 6: relationship to the activation of hypothalamic-pituitary-adrenal axis. Endocrinology 133:2523–2530
Acknowledgements
The Authors are grateful to the technical staff of the Department of Pharmacology and Toxicology, Faculty of Pharmacy, University of Benin, Benin City, Edo State, Nigeria.
Author information
Authors and Affiliations
Contributions
All authors contributed to this research work and the development of the final manuscript. EOTJ, BAB, OGA, and OIR designed the study and wrote the protocols; EOTJ, BAB, EEO, and AMA performed the experiments; EOTJ, AMA, and BAB did the ELISA study; EOTJ, BAB, AMA, UC, EEO, and OGA analyzed the data and literature searches; EOTJ, BAB, OGA, UC, and OIR contributed reagents/materials/analysis tools; EOTJ, BAB, OGA, and OGA. wrote the first draft of the manuscript; EOTJ, BAB, OGA, and OIR completed the final draft of the paper.
Corresponding authors
Ethics declarations
Ethics Approval
The experimental standard adopted for the study were approved and authorized according to the guidelines of the National Institute of Health for use of animals (NIH, 2016).
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Emudainohwo, J.O., Ben-Azu, B., Adebayo, O.G. et al. Normalization of HPA Axis, Cholinergic Neurotransmission, and Inhibiting Brain Oxidative and Inflammatory Dynamics Are Associated with The Adaptogenic-like Effect of Rutin Against Psychosocial Defeat Stress. J Mol Neurosci 73, 60–75 (2023). https://doi.org/10.1007/s12031-022-02084-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-022-02084-w