Abstract
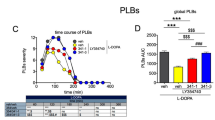
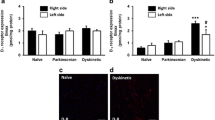
This study assessed striatal N-methyl-D-aspartate (NMDA) glutamate receptors of 1-methyl 4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) monkeys with levodopa (L-DOPA)-induced dyskinesias (LID). In a first experiment, four MPTP monkeys receiving L-DOPA/Benserazide alone developed dyskinesias. Four MPTP monkeys received L-DOPA/Benserazide plus CI-1041 an NMDA antagonist selective for NR1/NR2B and four were treated with L-DOPA/Benserazide plus a small dose of cabergoline; one monkey of each group developed mild dyskinesias at the end of treatment. In a second experiment, a kynurenine 3-hydroxylase inhibitor Ro 61-8048, combined with L-DOPA/Benserazide, reduced dyskinesias in MPTP monkeys. Drug-treated MPTP monkeys were compared to intact monkeys and saline-treated MPTP monkeys. Glutamate receptors were investigated by autoradiography using [3H]CGP-39653 (NR1/NR2A antagonist) and [3H]Ro25-6981 (NR1/NR2B antagonist). In general, striatal [3H]CGP-39653 specific binding was unaltered in all experimental groups. MPTP lesion decreased striatal [3H]Ro25-6981 specific binding; these levels were enhanced in the L-DOPA-alone-treated MPTP monkeys and decreased in antidyskinetic drugs treated monkeys. Maximal dyskinesias scores of the MPTP monkeys correlated significantly with [3H]Ro25-6981 specific binding in the rostral and caudal striatum. Hence, MPTP lesion, L-DOPA treatment and prevention of LID with CI-1041 and cabergoline, or reduction with Ro 61-8048 were associated with modulation of NR2B/NMDA glutamate receptors.







Similar content being viewed by others
Abbreviations
- DA:
-
Dopamine
- DAT:
-
Dopamine Transporter
- DOPAC:
-
3, 4 dihydroxyphenylacetic acid
- HVA:
-
Homovanillic acid
- L-DOPA:
-
Levodopa
- LID:
-
L-DOPA-induced dyskinesias
- LTD:
-
long term depression
- LTP:
-
long term potentiation
- 3-MT:
-
3-methoxytyramine
- NMDA:
-
N-methyl-D-aspartate
- PD:
-
Parkinson’s disease
References
Bara-Jimenez, W., Dimitrova, T. D., Sherzai, A., Aksu, M., & Chase, T. N. (2006). Glutamate release inhibition ineffective in levodopa-induced motor complications. Movement Disorders, 21, 1380–1383. doi:10.1002/mds.20976.
Barria, A., & Malinow, R. (2002). Subunit-specific NMDA receptor trafficking to synapses. Neuron, 35, 345–353. doi:10.1016/S0896-6273(02)00776-6.
Belanger, N., Gregoire, L., Hadj Tahar, A., & Bedard, P. J. (2003). Chronic treatment with small doses of cabergoline prevents dopa-induced dyskinesias in parkinsonian monkeys. Movement Disorders, 18, 1436–1441. doi:10.1002/mds.10589.
Blanchet, P. J., Calon, F., Morissette, M., et al. (2004). Relevance of the MPTP primate model in the study of dyskinesia priming mechanisms. Parkinsonism & Related Disorders, 10, 297–304. doi:10.1016/j.parkreldis.2004.02.011.
Bliss, T., & Schoepfer, R. (2004). Neuroscience. Controlling the ups and downs of synaptic strength. Science, 304, 973–974. doi:10.1126/science.1098805.
Brotchie, J. M., Lee, J., & Venderova, K. (2005). Levodopa-induced dyskinesia in Parkinson’s disease. Journal of Neural Transmission, 112, 359–391. doi:10.1007/s00702-004-0251-7.
Calabresi, P., Picconi, B., Parnetti, L., & Di Filippo, M. (2006). A convergent model for cognitive dysfunctions in Parkinson’s disease: the critical dopamine-acetylcholine synaptic balance. The Lancet Neurology, 5, 974–983. doi:10.1016/S1474-4422(06)70600-7.
Calon, F., Morissette, M., Ghribi, O., et al. (2002). Alteration of glutamate receptors in the striatum of dyskinetic 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated monkeys following dopamine agonist treatment. Progress in Neuro-Psychopharmacology & Biological Psychiatry, 26, 127–138. doi:10.1016/S0278-5846(01)00237-8.
Calon, F., Rajput, A. H., Hornykiewicz, O., Bedard, P. J., & Di Paolo, T. (2003). Levodopa-induced motor complications are associated with alterations of glutamate receptors in Parkinson’s disease. Neurobiology of Disease, 14, 404–416. doi:10.1016/j.nbd.2003.07.003.
Cepeda, C., Buchwald, N. A., & Levine, M. S. (1993). Neuromodulatory actions of dopamine in the neostriatum are dependent upon the excitatory amino acid receptor subtypes activated. Proceedings of the National Academy of Sciences of the United States of America, 90, 9576–9580. doi:10.1073/pnas.90.20.9576.
Chase, T. N. (2004). Striatal plasticity and extrapyramidal motor dysfunction. Parkinsonism & Related Disorders, 10, 305–313. doi:10.1016/j.parkreldis.2004.02.012.
Chen, L. R., Wesley, J. A., Bhattachar, S., Ruiz, B., Bahash, K., & Babu, S. R. (2003). Dissolution behavior of a poorly water soluble compound in the presence of Tween 80. Pharmaceutical Research, 20, 797–801. doi:10.1023/A:1023493821302.
Desce, J. M., Godeheu, G., Galli, T., et al. (1992). L-glutamate-evoked release of dopamine from synaptosomes of the rat striatum: involvement of AMPA and N-methyl-D-aspartate receptors. Neuroscience, 47, 333–339. doi:10.1016/0306-4522(92)90249-2.
Deutch, A. Y. (1993). Prefrontal cortical dopamine systems and the elaboration of functional corticostriatal circuits: implications for schizophrenia and Parkinson’s disease. Journal of Neural Transmission, 91, 197–221. doi:10.1007/BF01245232.
Dunah, A. W., Wang, Y., Yasuda, R. P., et al. (2000). Alterations in subunit expression, composition, and phosphorylation of striatal N-methyl-D-aspartate glutamate receptors in a rat 6-hydroxydopamine model of Parkinson’s disease. Molecular Pharmacology, 57, 342–352.
Erreger, K., Chen, P. E., Wyllie, D. J., & Traynelis, S. F. (2004). Glutamate receptor gating. Critical Reviews in Neurobiology, 16, 187–224. doi:10.1615/CritRevNeurobiol.v16.i3.10.
Erreger, K., Dravid, S. M., Banke, T. G., Wyllie, D. J., & Traynelis, S. F. (2005). Subunit-specific gating controls rat NR1/NR2A and NR1/NR2B NMDA channel kinetics and synaptic signalling profiles. The Journal of Physiology, 563, 345–358. doi:10.1113/jphysiol.2004.080028.
Erreger, K., Geballe, M. T., Kristensen, A., et al. (2007). Subunit-specific agonist activity at NR2A-, NR2B-, NR2C-, and NR2D-containing N-methyl-D-aspartate glutamate receptors. Molecular Pharmacology, 72, 907–920. doi:10.1124/mol.107.037333.
Gerkin, R. C., Lau, P. M., Nauen, D. W., Wang, Y. T., & Bi, G. Q. (2007). Modular competition driven by NMDA receptor subtypes in spike-timing-dependent plasticity. Journal of Neurophysiology, 97, 2851–2862. doi:10.1152/jn.00860.2006.
Gilgun-Sherki, Y., Melamed, E., Ziv, I., & Offen, D. (2003). Riluzole, an inhibitor of glutamatergic transmission, suppresses levodopa-induced rotations in 6-hydroxydopamine-lesioned rats. Pharmacology & Toxicology, 93, 54–56. doi:10.1034/j.1600-0773.2003.930108.x.
Gregoire, L., Rassoulpour, A., Guidetti, P., et al. (2008). Prolonged kynurenine 3-hydroxylase inhibition reduces development of levodopa-induced dyskinesias in parkinsonian monkeys. Behavioural Brain Research, 186, 161–167. doi:10.1016/j.bbr.2007.08.007.
Groc, L., Choquet, D., Stephenson, F. A., et al. (2007). NMDA receptor surface trafficking and synaptic subunit composition are developmentally regulated by the extracellular matrix protein Reelin. The Journal of Neuroscience, 27, 10165–10175. doi:10.1523/JNEUROSCI.1772-07.2007.
Hadj Tahar, A., Gregoire, L., Darre, A., et al. (2004). Effect of a selective glutamate antagonist on L-dopa-induced dyskinesias in drug-naive parkinsonian monkeys. Neurobiology of Disease, 15, 171–176. doi:10.1016/j.nbd.2003.10.007.
Hallett, P. J., Dunah, A. W., Ravenscroft, P., et al. (2005). Alterations of striatal NMDA receptor subunits associated with the development of dyskinesia in the MPTP-lesioned primate model of Parkinson’s disease. Neuropharmacology, 48, 503–516. doi:10.1016/j.neuropharm.2004.11.008.
He, L., Di Monte, D. A., Langston, J. W., & Quik, M. (2000). Autoradiographic analysis of N-methyl-D-aspartate receptor binding in monkey brain: effects of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine and levodopa treatment. Neuroscience, 99, 697–704. doi:10.1016/S0306-4522(00)00235-9.
Hilmas, C., Pereira, E. F., Alkondon, M., et al. (2001). The brain metabolite kynurenic acid inhibits alpha7 nicotinic receptor activity and increases non-alpha7 nicotinic receptor expression: physiopathological implications. The Journal of Neuroscience, 21, 7463–7473.
Hurley, M. J., Jackson, M. J., Smith, L. A., Rose, S., & Jenner, P. (2005). Immunoautoradiographic analysis of NMDA receptor subunits and associated postsynaptic density proteins in the brain of dyskinetic MPTP-treated common marmosets. The European Journal of Neuroscience, 21, 3240–3250. doi:10.1111/j.1460-9568.2005.04169.x.
Lavezzari, G., McCallum, J., Dewey, C. M., & Roche, K. W. (2004). Subunit-specific regulation of NMDA receptor endocytosis. The Journal of Neuroscience, 24, 6383–6391. doi:10.1523/JNEUROSCI.1890-04.2004.
Maura, G., Carbone, R., & Raiteri, M. (1989). Aspartate-releasing nerve terminals in rat striatum possess D-2 dopamine receptors mediating inhibition of release. The Journal of Pharmacology and Experimental Therapeutics, 251, 1142–1146.
Morissette, M., Dridi, M., Calon, F., et al. (2006). Prevention of levodopa-induced dyskinesias by a selective NR1A/2B N-methyl-D-aspartate receptor antagonist in parkinsonian monkeys: implication of preproenkephalin. Movement Disorders, 21, 9–17. doi:10.1002/mds.20654.
Moroni, F., Cozzi, A., Carpendo, R., et al. (2005). Kynurenine 3-mono-oxygenase inhibitors reduce glutamate concentration in the extracellular spaces of the basal ganglia but not in those of the cortex or hippocampus. Neuropharmacology, 48, 788–795. doi:10.1016/j.neuropharm.2004.10.019.
Nemeth, H., Toldi, J., & Vecsei, L. (2005). Role of kynurenines in the central and peripheral nervous systems. Current Neurovascular Research, 2, 249–260. doi:10.2174/1567202054368326.
Picconi, B., Centonze, D., Hakansson, K., et al. (2003). Loss of bidirectional striatal synaptic plasticity in L-DOPA-induced dyskinesia. Nature Neuroscience, 6, 501–506.
Picconi, B., Pisani, A., Barone, I., et al. (2005). Pathological synaptic plasticity in the striatum: implications for Parkinson’s disease. Neurotoxicology, 26, 779–783. doi:10.1016/j.neuro.2005.02.002.
Samadi, P., Gregoire, L., Morissette, M., et al. (2008). mGluR5 metabotropic glutamate receptors and dyskinesias in MPTP monkeys. Neurobiology of Aging, 29, 1040–1051. doi:10.1016/j.neurobiolaging.2007.02.005.
Samadi, P., Gregoire, L., Rassoulpour, A., et al. (2005). Effect of kynurenine 3-hydroxylase inhibition on the dyskinetic and antiparkinsonian responses to levodopa in Parkinsonian monkeys. Movement Disorders, 20, 792–802. doi:10.1002/mds.20596.
Samuel, D., Errami, M., & Nieoullon, A. (1990). Localization of N-methyl-D-aspartate receptors in the rat striatum: effects of specific lesions on the [3H]3-(2-carboxypiperazin-4-yl)propyl-1-phosphonic acid binding. Journal of Neurochemistry, 54, 1926–1933. doi:10.1111/j.1471-4159.1990.tb04893.x.
Schwab, R. S., England, A. C., Jr., Poskanzer, D. C., & Young, R. R. (1969). Amantadine in the treatment of Parkinson’s disease. Journal of the American Medical Association, 208, 1168–1170. doi:10.1001/jama.208.7.1168.
Schwarcz, R., & Pellicciari, R. (2002). Manipulation of brain kynurenines: glial targets, neuronal effects, and clinical opportunities. The Journal of Pharmacology and Experimental Therapeutics, 303, 1–10. doi:10.1124/jpet.102.034439.
Stone, T. W. (2000). Development and therapeutic potential of kynurenic acid and kynurenine derivatives for neuroprotection. Trends in Pharmacological Sciences, 21, 149–154. doi:10.1016/S0165-6147(00)01451-6.
Szabo, J., & Cowan, W. M. (1984). A stereotaxic atlas of the brain of the cynomolgus monkey (Macaca fascicularis). The Journal of Comparative Neurology, 222, 265–300. doi:10.1002/cne.902220208.
Thanvi, B., Lo, N., & Robinson, T. (2007). Levodopa-induced dyskinesia in Parkinson’s disease: clinical features, pathogenesis, prevention and treatment. Postgraduate Medical Journal, 83, 384–388. doi:10.1136/pgmj.2006.054759.
Tovar, K. R., & Westbrook, G. L. (2002). Mobile NMDA receptors at hippocampal synapses. Neuron, 34, 255–264. doi:10.1016/S0896-6273(02)00658-X.
Ulas, J., Weihmuller, F. B., Brunner, L. C., et al. (1994). Selective increase of NMDA-sensitive glutamate binding in the striatum of Parkinson’s disease, Alzheimer’s disease, and mixed Parkinson’s disease/Alzheimer’s disease patients: an autoradiographic study. The Journal of Neuroscience, 14, 6317–6324.
Acknowledgements
This research was funded by a grant from the Canadian Institutes of Health Research to TDP. BO was supported by the Government of Ivory Coast and SB by the Government of Tunisia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ouattara, B., Belkhir, S., Morissette, M. et al. Implication of NMDA Receptors in the Antidyskinetic Activity of Cabergoline, CI-1041, and Ro 61-8048 in MPTP Monkeys with Levodopa-induced Dyskinesias. J Mol Neurosci 38, 128–142 (2009). https://doi.org/10.1007/s12031-008-9137-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-008-9137-8