Abstract
Purpose
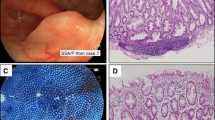
The aims of this study are to characterize the frequency, density, and distribution of aberrant crypt foci (ACF) and its histological features and to determine the frequency of loss of expression of DNA mismatch repair (MMR) proteins of subjects with hereditary nonpolyposic colorectal cancer (HNPCC) and sporadic colon rectal cancer (CRC).
Methods
Patients with HNPCC, first-degree relatives of subjects with HNPCC, sporadic CRC, and average risk subjects of sporadic CRC were included prospectively. Total colonoscopy with chromoendoscopy using methylene blue 0.5% and magnification in the right colon (cecum and 20 cm of the ascending colon) and in the left colon (rectum) was performed; loss of expression of MLH1 and MSH2 was evaluated by immunohistochemistry in confirmed ACF.
Results
Fifty-two subjects were included. Thirty-eight of the 119 ACF detected by endoscopy were biopsied. In 14 of the 38 specimens (36.8%), ACF were confirmed by histology (Cohen's kappa, 0.44). In subjects with HNPCC, ACF were identified more frequently in the right segment of the colon than in the left (73.1% vs. 26%); in contrast, ACF predominated in the left segment of the colon (89.3% vs. 10.6%) in subjects with sporadic CRC. There was a loss of MLH1 expression in ACF in subjects with HNPCC.
Conclusions
In HNPCC, we found a greater density of ACF in the right colon, and in sporadic CRC, greater density in the left. ACF present loss in the expression of DNA MMR protein and can be used as an early marker in patients with a risk of HNPCC in whom carcinogenesis appears to be accelerated.



Similar content being viewed by others
References
Jemal A, Bray F, Center MM, et al. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69–90.
Boyle P, Leon ME. Epidemiology of colorectal cancer. Br Med Bull. 2002;64:1–25.
DeFrancisco J, Grady WM. Diagnosis and management of hereditary non-polyposis colon cancer. Gastrointest Endosc. 2003;58:390–408.
Giardiello FM, Brensinger JD, Petersen GM. AGA technical review on hereditary colorectal cancer and genetic testing. Gastroenterology. 2001;121:198–213.
Lubinski J, Lynch HT, Lynch J, et al. HNPCC (Lynch syndrome): differential diagnosis, molecular genetics and management—a review. Hered Cancer Clin Prac. 2003;1:7–18.
Liu B, Parsons R, Papadopoulos N, et al. Analysis of mismatch repair genes in hereditary non-polyposis colorectal cancer patients. Nat Med. 1996;2:169–74.
Boland CR, Koi M, Chang DK, et al. The biochemical basis of microsatellite instability and abnormal immunohistochemistry and clinical behavior in Lynch syndrome: from bench to bedside. Fam Cancer. 2008;7:41–52.
Piñol V, Castells A, Andreu M, et al. Accuracy of revised Bethesda guidelines, microsatellite instability, and immunohistochemistry for the identification of patients with hereditary nonpolyposis colorectal cancer. JAMA. 2005;293:1986–94.
Müller A, Giuffre G, Edmonston TB, et al. Challenges and pitfalls in HNPCC screening by microsatellite analysis and immunohistochemistry. J Mo Diagn. 2004;6:308–15.
Overbeek LI, Ligtenberg MJ, Willems RW, et al. Interpretation of immunohistochemistry for mismatch repair proteins is only reliable in a specialized setting. Am J Surg Pathol. 2008;32:1246–51.
Lynch HT, Smyrk TC, Watson P, et al. Genetics, natural history, tumor spectrum, and pathology of hereditary nonpolyposis colorectal cancer: an updated review. Gastroenterology. 1993;104:1535–49.
Church J. Hereditary colon cancers can be tiny: a cautionary case report of the results of colonoscopic surveillance. Am J Gastroenterol. 1998;93:2289–90.
Morson BC. Evolution of cancer of the colon and rectum. Cancer. 1974;34:845–9.
Winawer SJ, Zauber AG, Ho MN, et al. Prevention of colorectal cancer by colonoscopic polypectomy. The National Polyp Study Workgroup. N Engl J Med. 1993;329:1997–81.
Citarda F, Tomaselli G, Capocaccia R, et al. Efficacy in standard clinical practice of colonoscopic polypectomy in reducing colorectal cancer incidence. Gut. 2001;48:812–5.
Davila RE, Rajan E, Baron TH, et al. ASGE guideline: colorectal cancer screening and surveillance. Gastrointest Endosc. 2006;63:546–57.
Bird RP. Observation and quantification of aberrant crypts in the murine colon treated with a colon carcinogen: preliminary finding. Cancer Lett. 1987;37:147–51.
Pretlow TP, Barrow BJ, Ashton WS, et al. Aberrant crypts: putative preneoplasic foci in human colonic mucosa. Cancer Res. 1991;51:1564–7.
Roncucci L, Pedroni M, Vaccina F, et al. Aberrant crypt foci in colorectal carcinogenesis. Cell and crypt dynamics. Cell Prolif. 2000;33:1–18.
Alrawi SJ, Schiff M, Carroll RE, et al. Aberrant crypt foci. Anticancer Res. 2006;26:107–19.
Cheng L, Lai MD. Aberrant crypt foci as microscopic precursors of colorectal cancer. World J Gastroenterol. 2003;9:2642–9.
Hurlstone DP, Cross SS. Role of aberrant crypt foci detected using high-magnification-chromoscopic colonoscopy in human colorectal carcinogenesis. J Gastroenterol Hepatol. 2005;20:173–81.
Yamashita N, Minamoto T, Ochiai A, et al. Frequent and characteristic K-ras activation in aberrant crypt foci of colon: is there preference among K-ras mutants for malignant progression? Cancer. 1995;75:1527–33.
Pedroni M, Sala E, Scarselli A, et al. Microsatellite instability and mismatch-repair protein expression in hereditary and sporadic colorectal carcinogenesis. Cancer Res. 2001;61:896–9.
Nucci MR, Robinson CR, Longo P, et al. Phenotypic and genotypic characteristics of aberrant crypt foci in human colorectal mucosa. Hum Pathol. 1997;28:1396–407.
Di Gregorio C, Losi L, Fante R, et al. Histology of aberrant crypt foci in the human colon. Histopathology. 1997;30:328–34.
Takayama T, Katsuki S, Takahashi Y, et al. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl J Med. 1998;339:1277–84.
Heinen CD, Shivapurkar N, Tang Z, et al. Microsatellite instability in aberrant crypt foci from human colons. Cancer Res. 1996;56:5339–41.
Adler DG, Gostout CJ, Sorbi D, et al. Endoscopic identification and quantification of aberrant crypt foci in the human colon. Gastrointest Endosc. 2002;56:657–62.
Mutch MG, Schoen RE, Fleshman JW, et al. A multicenter study of prevalence and risk factors for aberrant crypt foci. Clin Gastroenterol Hepatol. 2009;7:568–74.
Acknowledgements
The authors would like to thank Luis Alonso Herrera Montalvo PhD, Cancer Biomedical Research Unit of the UNAM/INCan, for the support to complete this investigation.
Disclosure
The authors report that there are no disclosures to this publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ramírez-Ramírez, M.A., Sobrino-Cossío, S., de la Mora-Levy, J.G. et al. Loss of Expression of DNA Mismatch Repair Proteins in Aberrant Crypt Foci Identified In Vivo by Magnifying Colonoscopy in Subjects with Hereditary Nonpolyposic and Sporadic Colon Rectal Cancer. J Gastrointest Canc 43, 209–214 (2012). https://doi.org/10.1007/s12029-011-9303-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12029-011-9303-z