Abstract
Background
To identify a reliable method of performing apnea testing as part of brain death determination in adult patients who develop loss of brainstem reflexes while receiving extracorporeal membrane oxygenation (ECMO). ECMO provides extracirculatory support to patients in cardiorespiratory failure who would otherwise be expected to die. Many studies have reported brain death as a potential complication of adult ECMO, but none have cited how apnea testing was performed in these patients.
Methods
This retrospective review identified adults 15 years or older treated with ECMO at our institution (2002–2010) and the method of determination of brain death when complete loss of brainstem reflexes occurred.
Results
Loss of all brainstem reflexes was identified in three cases (3/87, 3.4%). The apnea test was not performed since it was deemed “difficult,” leading to withdrawal of ECMO and intensive care. Ancillary tests such as cerebral flow studies were not used because they may not document absent cerebral arterial flow due to the ischemic nature of the injury. We propose the use of an oxygenated apnea test on ECMO using continuous positive airway pressure (CPAP) through the ventilator or anesthesia bag, with an inline manometer and an end tidal CO2 device.
Conclusion
Apnea testing is essential in the determination of brain death, but may not be employed in ECMO-treated adult patients. Apnea testing using the above protocol may assist in better decision making for adult ECMO patients at risk of brain death.
Similar content being viewed by others
Introduction
Extracorporeal membrane oxygenation (ECMO) is increasingly used as a means of extracirculatory support to patients in severe, reversible cardiac and/or respiratory failure. ECMO has been found to improve the chances of survival in some children and adults, including patients who undergo cardiopulmonary resuscitation with failure to restore circulation (E-CPR) [1]. ECMO, however, carries a risk of neurological injury related to both the precipitating event that led to ECMO and ECMO therapy itself. In 2009, the national Extracorporeal Life Support Organization (ELSO) registry reported that 21% of 295 adults treated with E-CPR experienced brain death. Patients presumed to have brain death had sooner withdrawal of ECMO support [1]. In 147 pediatric patients with brain injury, a separate report found the incidence of neurological injury was 22% with 11% of these patients experiencing brain death [2]. Although these studies report brain death as a potential sequela in patients who receive ECMO, none have cited how apnea testing was performed. The objective of this study is to propose a method of performing apnea testing, as part of the adult brain death examination, in patients without brainstem reflexes receiving ECMO.
Methods
A retrospective cohort study was performed at the Mayo Clinic (Rochester, MN) of all patients treated with veno-venous or veno-arterial ECMO between January 2002–May 2010 [3]. Patients ages 15 years old and above at the time of ECMO treatment were included if they experienced loss of all brainstem reflexes (light-fixed pupils, absent corneal reflex, absent cough to tracheal suctioning, absent pharyngeal reflex (gag), absent oculocephalic responses with 30-ml iced water irrigation of the ear canals, and absent motor response to pain).
There are no set criteria for choosing adults for ECMO treatment at this institution. The decision for a trial of ECMO is usually made by the cardiothoracic surgeon and/or anesthesiologist involved in the patient’s care. Exclusionary criteria include evidence of a preceding catastrophic neurological event or irreversible heart or lung injury.
Patients with loss of brainstem reflexes were found through a retrospective review of the adult ECMO registry. Medical records, including clinical evaluation notes, intra-operative monitoring reports, laboratory results, brain imaging studies, electrophysiological studies, and pathological specimens from cerebral autopsy were reviewed. The Mayo Clinic Institutional Review Board approved this study.
Results
Three patients were found to have loss of all brainstem reflexes. Clinical variables of the three patients are summarized in Table 1.
Case 1
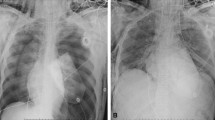
A 32-year-old man with advanced aortic stenosis experienced witnessed ventricular fibrillation cardiac arrest, treated with 12 min of CPR. He underwent emergent aortic valve replacement. His postoperative course was remarkable for cardiogenic shock, necessitating ECMO placement. His neurological examination was normal at the time of ECMO initiation. Shortly afterward, he developed a dilated left pupil, followed by loss of all brainstem reflexes in the setting of normothermia, absence of CNS depressant agents, and systolic blood pressure greater than 90 mm Hg. Computed tomography of the head showed large multilobar intraparenchymal hemorrhages with marked sulcal, ventricular and bibasilar cisternal effacement, subfalcine herniation, and 1 cm of midline shift (Fig. 1a). Electroencephalogram (EEG) demonstrated electrocerebral silence (2 microvolts). Apnea testing was deemed too difficult to perform. The patient was declared brain dead 34 h after initiation of ECMO. Neuropathological findings on autopsy showed intracerebral and Duret hemorrhages, hypoxic-ischemic injury with acute “red dead neurons,” cerebral edema, bilateral cerebellar tonsillar herniation with necrosis and fragmentation, and superimposed brain death syndrome.
Case 2
A 69-year-old woman with rheumatic multivalvular heart disease, chronic atrial fibrillation, and nicotine dependence underwent mitral valve replacement, tricuspid valve repair, and single-vessel coronary artery bypass grafting. This was complicated by prolonged pump time and coagulation abnormalities. Her postoperative course was remarkable for cardiogenic shock, multiorgan failure, severe lactic acidosis, and disseminated intravascular coagulopathy (DIC). She required several mediastinal explorations during which she was placed on ECMO and intra-aortic balloon pump for cardiopulmonary support. She continued to deteriorate despite multiple transfusions, vasopressor augmentation and hemodialysis, and was noted to be unresponsive with fixed pupils off sedation 2.4 days after ECMO initiation. Neurology consultation documented loss of all brainstem reflexes and lack of spontaneous chest rise on ECMO. CT head could not be performed due to her unstable clinical condition. A repeat neurological examination was recommended to completely exclude effect of sedation, but life support was withdrawn 4 h later. Autopsy was refused.
Case 3
A 50-year-old man with testicular cancer, renal allograft transplant on chronic immunosuppression, status post resection of left total hip arthroplasty secondary to Streptococcus viridans infection, underwent removal of a left hip articulating antibiotic cement spacer and reimplantation of a left total hip arthroplasty without complications. Immediately postoperatively, he developed cardiopulmonary arrest in the setting of pulmonary embolism and required several minutes of CPR, and then vasopressor support, continuous heparin infusion, and ECMO placement. Echocardiogram showed moderate to severe right ventricular enlargement and systolic dysfunction, hyperdynamic left ventricular systolic function, and decreased left ventricular cavity size. He was noted to have fixed dilated pupils and lack of spontaneous movement shortly after ECMO placement. Neurology consultation documented loss of all brainstem reflexes 20.5 h after ECMO initiation. CT scan showed severe diffuse brain edema with effacement of the basilar cisterns, and third and fourth ventricles, suggestive of severe diffuse anoxic brain injury (Fig. 1b). Confirmatory testing with EEG revealed electrocerebral silence. Apnea testing was deemed difficult to perform. Autopsy was refused.
Discussion
Irreversible coma with a known cause, absence of brain stem reflexes and apnea, constitutes a diagnosis of brain death according to the American Academy of Neurology (AAN) [4]. Apnea testing is a key component of brain death testing as its presence demonstrates medullary dysfunction [5]. An apnea test was not performed in these three patients with presumed brain death due to lack of standardized protocols. This often leads to the use of confirmatory tests including EEG, which have questionable utility in confirming brain death [4] or withdrawal of support. A practice of early withdrawal of support unfortunately limits the possibility of organ donation in patients who would otherwise be declared brain dead. We therefore suggest the following procedure to perform an oxygenated apnea test on ECMO.
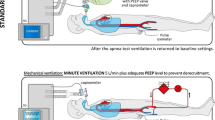
Prerequisites include elimination of the influence of neuromuscular blocking agents and central nervous system-depressant agents. Caution must be exercised in cases with hepatic or renal failure and in hypothermia where metabolism of such drugs may become impaired and the effects prolonged. Acid–base and severe electrolyte disturbances must also be excluded. Body temperature should be at least 36° centigrade and warming blankets may be used if needed. Pretest systolic blood pressure of ≥100 mmHg should be maintained through the ECMO circuit, with or without the use of vasopressors or inotropes. The patient should be placed on adequate continuous positive airway pressure (CPAP) through the ventilator or anesthesia bag, with an inline manometer and an end tidal CO2 device to aid in the detection of respiration. Though CPAP is used by many people, an anesthesia bag (a simple thick balloon with a valve) is very easy to understand, sensitive to observe breathing, does not require turning off apnea alarms, and can eliminate any question of any influence by the ventilator. Levesque et al. report favorable apnea testing with CPAP, and PaO2 was best maintained by this method compared to T-piece oxygen delivery (12 l/min) and oxygen catheter inserted through the endotracheal tube (6 l/min).[6] One hundred percent oxygen should be provided via ventilator, anesthesia bag, and/or through the ECMO blender circuit at the lowest oxygenator sweep flow possible to maintain >90% oxygen saturation on pulse oximetry for at least 8 min. Preoxygenation promotes cellular metabolism, resulting in rapid formation of PaCO2 in the cerebrospinal fluid which stimulates respiratory centers in the medulla oblongata [7, 8]. A baseline arterial blood gas should be obtained either through the patient’s arterial line or periphery to confirm eucapnia (PaCO2 35–45 mm Hg), before addition of CO2 to the oxygenator to a target of 60 mm Hg or ≥20 mmHg from the baseline normal CO2 value. Monitor closely for respiratory movements, keeping in mind complex sequential movements secondary to ischemic spinal cord neurons may be observed and can be consistent with a brain death diagnosis.[4, 5, 8, 9] If apnea is observed during the 8-min observation interval, the patient is dead and a repeat arterial blood gas should be performed
Conclusion
Apnea testing is an important part of brain death determination but presents unique challenges in patients receiving ECMO. It is not employed consistently in ECMO-treated patients due to a lack of standardized and accepted procedures. A timely diagnosis of brain death is important for families and ICU resource utilization. In addition, the opportunity for organ donation may be preserved when brain death is diagnosed promptly. This article describes a valid protocol for accurate apnea testing in patients presumed brain dead who are receiving ECMO.
References
Thiagarajan RR, et al. Extracorporeal membrane oxygenation to support cardiopulmonary resuscitation in adults. Ann Thorac Surg. 2009;87(3):778–85.
Barrett CS, et al. Neurological injury after extracorporeal membrane oxygenation use to aid pediatric cardiopulmonary resuscitation. Pediatr Crit Care Med. 2009;10(4):445–51.
Mateen FJ, Muralidharan R, Shinohara RT, et al. Neurological disorders and survival of 87 adults on extra-corporeal membrane oxygenation (ECMO): a single institution cohort study. In: Abstract Poster 117. Neurocritical care Society, 8th annual meeting, San Francisco, CA, 16 Sept 2010.
Wijdicks EF, et al. Evidence-based guideline update: determining brain death in adults: report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 2010;74(23):1911–8.
Ropper AH, Kennedy SK, Russell L. Apnea testing in the diagnosis of brain death. Clinical and physiological observations. J Neurosurg. 1981;55(6):942–6.
Levesque S, et al. Efficacy of a T-piece system and a continuous positive airway pressure system for apnea testing in the diagnosis of brain death. Crit Care Med. 2006;34(8):2213–6.
Goudreau JL, Wijdicks EF, Emery SF. Complications during apnea testing in the determination of brain death: predisposing factors. Neurology. 2000;55(7):1045–8.
Wijdicks EF, et al. Pronouncing brain death: contemporary practice and safety of the apnea test. Neurology. 2008;71(16):1240–4.
Ropper AH. Unusual spontaneous movements in brain-dead patients. Neurology. 1984;34(8):1089–92.
Conflicts of interest
All authors have no conflicts of interest related to this study.
Disclosures
Dr. Wijdicks is editor-in-chief of Neurocritical Care. Drs. Muralidharan, Mateen, and Schears and Mr. Shinohara have no disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muralidharan, R., Mateen, F.J., Shinohara, R.T. et al. The Challenges with Brain Death Determination in Adult Patients on Extracorporeal Membrane Oxygenation. Neurocrit Care 14, 423–426 (2011). https://doi.org/10.1007/s12028-011-9516-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-011-9516-9