Abstract
Purpose
To evaluate the feasibility and efficacy of an apnea test (AT) technique that combines the application of positive end expiratory pressure (PEEP) with subsequent pulmonary recruitment in a large cohort of brain-dead patients.
Methods
This study was a retrospective analysis of prospectively collected data on brain-dead patients admitted to our institution (Hospital San Gerardo, Monza, Italy) between January 2010 and December 2014. The rate of aborted apnea tests (ATs), occurrence of complications (i.e., pneumothorax, cardiac arrhythmias, cardiac arrest, and severe hypoxia, defined as PaO2 < 40 mmHg), ventilator settings, hemodynamics, and blood gas analyses were evaluated. Subgroup analysis was performed, with patients classified into veno-arterial extracorporeal membrane oxygenation (ECMO) or non-ECMO groups, and into hypoxic (i.e., baseline PaO2/FiO2 < 200 mmHg) and non-hypoxic (i.e., baseline PaO2/FiO2 > 200 mmHg) groups.
Results
In total, 169 consecutive patients including 25 on ECMO were included in the study. No AT abortion nor severe complications were detected. The AT was completed in all patients. Fluid boluses and increases or initiation of vasoactive drugs were required in less than 10 and 3 % of the AT procedures, respectively. No clinically meaningful alteration in hemodynamics was recorded. Severe hypoxia occurred during 7 (2.4 %) and 4 (8 %) of the ATs performed in non-ECMO and ECMO patients, respectively (p = 0.063), and it occurred more frequently in hypoxic patients than in non-hypoxic patients (11.1 vs. 4.8 %, respectively; p = 0.002).
Conclusions
In a large cohort of consecutive patients, including the largest patient population on ECMO reported to date, our AT technique that combines the application of PEEP with subsequent pulmonary recruitment proved to be feasible and safe.
Similar content being viewed by others
Introduction
The apnea test (AT) is a key component in the clinical determination of brain death (BD) [1, 2]. The AT result is considered to be positive if respiratory movements are absent and arterial carbon dioxide partial pressure (PaCO2) is >60 mmHg, with an increase of >20 mmHg from baseline. The AT is a low-volume procedure, with wide practice variability [3, 4] and a low evidence of best practice [5, 6]. A number of complications are associated with the AT, including hypoxemia, ranging between 4 and 25 %, and hypotension. The rate of aborted ATs has been reported to be 3 %. In the past, AT was performed by delivering oxygen through a simple tube into the endotracheal tube; however, serious complications (i.e., barotrauma, pneumothorax, air trapping) were associated with this procedure [7–12]. Alternatively, oxygenation can be provided to the subject through a T-piece system with a fresh oxygen flow of around 6–10 L/min.
An even more challenging scenario is performing the AT in patients supported by extracorporeal membrane oxygenation (ECMO). Small series dealing with this particular clinical setting have been recently published [13–16]. In patients connected to veno-arterial ECMO (VA-ECMO), the AT is performed by decreasing the sweep gas flows (GF) to 1 L/min while increasing the fraction of inspired oxygen to the membrane lung (FiO2ML) up to 100 % [17–20].
In our institution, in order to avoid de-recruitment and hypoxia, we apply routinely positive end expiratory pressure (PEEP) during the AT and perform recruitment maneuvers thereafter. This approach is employed in both non-ECMO and ECMO patients. Here, we describe our clinical experience with this technique in a large cohort of consecutive patients undergoing BD assessment. The primary aims of this study were to evaluate the rate of aborted ATs, the incidence of complications, and the impact of the AT on oxygenation. Our secondary aim was to assess these variables in patients on VA-ECMO support.
Methods
This is a retrospective analysis of data prospectively collected from all patients admitted to the intensive care units (ICUs) (i.e., Neurocritical, General, Cardiac–surgical) of San Gerardo Hospital (Monza, Italy) between 2010 and 2014. The Institutional Ethical Committee approved the study protocol on 27 November 2014 and the requirement for written informed consent was waived. The inclusion criteria were: (1) legal status of BD as determined according to Italian legislation [21] and confirmed by a search of the Lombardy online BD registry; (2) age ≥18 years.
According to the Italian law, the determination of BD requires a 6-h legal observation period. The baseline diagnosis of BD is based upon coma status with absent brainstem reflexes, absence of spontaneous breathing as documented by a AT with a PaCO2 of ≥60 mmHg and pH <7.40, and the absence of cortical electrical activity for 30 min on a electroencephalogram (EEG). At the beginning and at the end of this observation period, a full neurological examination, a 30-min EEG recording, and an AT are performed.
In accordance with Italian law, compulsory arterial blood gas analyses at the time of BD diagnosis and twice during the observation period are performed. In our institution, we also perform arterial blood gas analyses after each of the ATs. To evaluate the clinical effects of the AT on blood gases, we identified five time-points:
-
1.
baseline diagnosis of BD (baseline);
-
2.
end of the first AT performed during the observation period (1st AT);
-
3.
after first AT (not mandatory);
-
4.
end of the second AT performed during the observation period (2nd AT);
-
5.
after second AT (not mandatory).
At each time-point we recorded ventilatory mode (i.e., volume or pressure-controlled); ventilator settings (i.e., FiO2, PEEP, respiratory rate, tidal volume, mean airway pressure, peak inspiratory pressure); ECMO settings, if applicable [i.e., blood flow (BF), GF, FiO2ML]; blood gas analyses [i.e., pH, PaCO2, arterial oxygen partial pressure (PaO2)]; PaO2/FiO2 ratio.
At our institution, the ATs are carried out as follows (see Fig. 1):
-
Before the test, the respiratory rate is titrated to obtain a PaCO2 of 40–45 mmHg; low tidal volumes (i.e., 6–8 mL/kg of predicted body weight) are used plus a periodic hyperinflation of the lungs (SIGH) every 2 min (peak pressures of 40 cm H2O, 4–5 s), whereas FiO2 and PEEP level are adjusted to obtain PaO2 > 90 mmHg. In previously documented hypercapnic patients (such as patients with chronic pneumopathies and/or chronic metabolic alkalosis), pH rather than PaCO2 normalization is targeted.
-
A short pre-oxygenation period with 100 % FiO2 is started 5 min before the AT.
-
During the AT, the patient is disconnected from the ventilator, and the endotracheal tube is connected to a resuscitator bag (VBM Medizintechnik GmbH, Sulz am Neckar, Germany) providing 8 L/min of oxygen. An adjustable PEEP valve (VBM Medizintechnik GmbH, Sulz am Neckar, Germany) is connected to the resuscitator bag and set to provide the same PEEP level used during mechanical ventilation; SpO2 (peripheral capillary O2 saturation) and invasive arterial pressure are monitored continuously.
-
In all the patients, including previously documented hypercapnic patients, an increase of 20 mmHg over baseline is targeted.
-
After the AT, patients are reconnected to the ventilator with the same settings as those in use before the test. Recruitment maneuvers are performed after the AT if the oxygen saturation is <92 %.
Depiction of the procedures utilized by our institution (Hospital San Gerardo, Monza, Italy) to carry out apnea tests (ATs). FiO 2 Fraction of inspired O2, PaO 2 arterial oxygen partial pressure, PaCO 2 partial pressure of carbon dioxide, PEEP positive end expiratory pressure, ECMO extracorporeal membrane oxygenation. Figure by S. Sosio, MD
In patients supported by ECMO, a similar procedure is carried out and ECMO is managed as follows:
-
During the AT, the extracorporeal BF is not modified, GF is reduced to 1 L/min, and FiO2ML is increased to 100 %.
-
After the AT, GF, and FiO2ML are returned to baseline values.
An intensivist performs all tests in presence of a neurologist and a legal medicine specialist. Abortion of the AT based on hypoxia is at the discretion of the attending physician.
Data collection for this study is described in detail in the Electronic Supplementary Material (ESM) Additional Methods, Data Collection.
In our study, patients supported by ECMO at the time of BD determination were defined as “ECMO patients” and those not supported by ECMO as “non-ECMO” patients. Notably, due to the overwhelming impact of ECMO on oxygenation, the effects of AT on the PaO2/FiO2 of ECMO patients were not studied. We further characterized non-ECMO subjects as “hypoxemic” if their baseline PaO2/FiO2 was <200 mmHg and “non-hypoxemic” if it was >200 mmHg.
Statistical analysis
Data are presented as the mean ± standard deviation or as the median and interquartile range (IQR), where appropriate. A two-way repeated-measures analysis of variance with time-points (5 levels) and patient categories (i.e., ECMO vs. non-ECMO or hypoxemic vs. non-hypoxemic; 2 levels) as fixed effects and subjects as random effects was performed using the general linear model. The post-hoc Student’s t test with Tukey’s adjustment was used for multiple comparisons. The Fisher’s Exact Test was used to analyze dichotomous categorical variables. Two-tailed values of p of <0.05 were deemed statistically significant. The JMP version 11 statistical program (SAS, Cary, NC) and Sigmaplot version 12.0 (Systat Software, San Jose, CA) were used for the statistical analyses and graph compilation, respectively.
Results
A total of 170 patients underwent legal BD determination during the study period in the three ICUs participating in this study (see ESM Fig. S1, Additional Results). One patient was excluded from the analysis due to an age of <18 years; thus 169 patients were enrolled in the analysis. Of these 169 patients, 25 were provided with VA-ECMO support during the BD assessment. A single patient died between the first and second ATs due to cardiac arrhythmias leading to cardiac arrest; all of the other patients successfully underwent both ATs. Table 1 shows the demographics and the baseline parameters of the patients' population prior to conducting the BD assessment. Compared to the non-ECMO subgroup, patients in the ECMO subgroup were more frequently male (76 vs. 52 %; p = 0.003), heavier (80 vs. 70 kg; p = 0.003), and younger (58 vs. 69 years; p < 0.001). Length of ICU stay was higher in the ECMO subgroup. Of the 25 ECMO patients, 23 (92 %) were connected to the ECMO system after cardiac arrest, one was connected for cardiogenic shock, and one due to post-cardiotomy shock. Only the latter was centrally cannulated, while peripheral cannulation was used in all other patients. Intracranial hemorrhage was the main cause of death in the non-ECMO subgroup (50 % of patients), whereas in ECMO patients the most frequent cause was postanoxic encephalopathy (84 %). For further details on ECMO patients, see ESM Additional Results, ECMO Details.
The median OTO score [22] was 7 in both the ECMO and non-ECMO patients. Lungs were harvested in 9 % of non-ECMO patients and in 16 % of ECMO patients (p = 0.28).
Volume-controlled ventilation was used in 85 % of patients in the non-ECMO subgroup, while pressure-controlled ventilation was used most frequently (92 %) in the ECMO subgroup. Compared to non-ECMO patients, ECMO patients were ventilated with a higher FiO2 (60 vs. 50 %; p < 0.001), PEEP (10 vs. 5 cmH2O; p < 0.001), tidal volume (600 vs. 472 mL; p < 0.001) and peak airway pressure (25 vs. 17 cmH2O; p < 0.001), while respiratory rate was lower (8 vs. 12 bpm; p < 0.001). Baseline blood gases were similar between the two subgroups: median PaO2, PaCO2, and pH were 129 mmHg, 43 mmHg, and 7.41, respectively, in non-ECMO patients and 122 mmHg, 45 mmHg, and 7.41, respectively, in ECMO patients. Half of non-ECMO patients had a PaO2/FiO2 ratio of >300 mmHg, whereas only two patients had a PaO2/FiO2 ratio of <100 mmHg. Median duration of ECMO treatment, BF, GF, and FiO2ML are given in Table 1.
No interruption of the AT was recorded. Pneumothorax, pneumomediastinum, cardiac arrhythmias, and cardiac arrest did not occur in any patient.
Hemodynamic effects of the AT are shown in Table 2. The heart rate was similar in ECMO and non-ECMO patients (p = 0.903), while ECMO patients had a lower mean arterial pressure (p < 0.001). Non-ECMO patients showed a statistically significant, albeit clinically meaningless, increase in heart rate at the end of the ATs (p < 0.001); in contrast, ECMO patients had a stable heart rate during the ATs (p = 0.475). Mean arterial pressure was not altered by the AT in either the ECMO (p = 0.264) or non-ECMO patients (p = 0.244). Overall, fluid boluses and increases or starting of vasoactive drug therapy were necessary in less than 10 and 3 %, respectively, of the AT procedures. ECMO and non-ECMO patients did not differ with regards to the need for hemodynamic interventions.
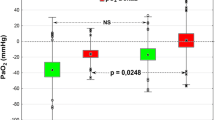
Figure 2 shows the arterial PaO2, PaCO2 and pH before, during and after the ATs. All patients met the legal criteria for BD determination (PaCO2 > 60 mmHg or increase in PaO2 > 20 mmHg; pH < 7.4) during the ATs. PaO2 was higher after the first and the second AT compared to other time-points. In non-ECMO patients, FiO2 was 100 % during the AT, whereas it was 50 % (40–50 %), 70 % (50–100 %), and 60 % (50–90 %) at baseline, after the first AT, and after the second AT, respectively (p < 0.001). In the non-ECMO subgroup, the PaO2/FiO2 ratio before and after the ATs did not undergo any significant variation, and the median PaO2/FiO2 ratio was 301, 315, and 287 mmHg at baseline, after the first AT, and after the second AT, respectively (p = 0.76; see Fig. 3).
Box and whisker plots of arterial blood gas analyses during brain death assessment in no-ECMO (white boxes) and ECMO (blue boxes) patients. Data are presented as the median (horizontal line in box) with interquartile range (top and bottom of box) and 10th and 90th percentiles (whiskers). Open circles Outliers. Steps 1–5 Steps involved in the determination of brain death (see List in "Methods"): Step 1 baseline, Step 2 end of the first AT, Step 3 after the first AT, Step 4 end of the second AT, Step 5 after the second AT. Two-way analysis of variance reporting differences in blood gas analyses due to treatment (i.e., ECMO vs. no-ECMO) or experimental step. *p < 0.05 vs. no-ECMO patients. Numbers 1–5 above datasets 1 p < 0.05 vs. Step 1, 2 p < 0.05 vs. Step 2, 3 p < 0.05 vs. Step 3, 4 p < 0.05 vs. Step 4, 5 p < 0.05 vs. Step 5
Box and whisker plot of the PaO2/FiO2 ratio of no-ECMO patients at baseline (Step 1), after the first AT (Step 3), and after the second AT (Step 5). Data are presented as the median (horizontal line in box) with interquartile range (top and bottom of box) and 10th and 90th percentiles (whiskers). Open circles Outliers
Of the non-ECMO patients, 24 % had a baseline PaO2/FiO2 ratio of <200 mmHg and were categorized as hypoxemic. In this subgroup, PaO2 at the end of the apnea period was lower than that of non-hypoxemic patients [79 (IQR 63–108) mmHg vs. 154 (IQR 79–288) mmHg; p < 0.001]. PaCO2 and pH did not differ between hypoxemic patients and non-hypoxemic patients: 68 (IQR 64–75) versus 68 (IQR 64–74) mmHg (p = 0.86) and 7.25 (IQR 7.2–7.28) versus 7.25 (IQR 7.22–7.28) (p = 0.17), respectively.
Severe hypoxia (i.e., PaO2 < 40 mmHg) was detected at the end of 11 (3.2 % of all ATs performed) ATs: six (3.55 %) at the end of the first AT and five (2.9 %) of the end of the second AT. A single patient had severe hypoxia at the end of both ATs. The incidence of severe hypoxia at the end of at least one of the ATs was 11.1 and 18 % in hypoxemic and non-hypoxemic patients, respectively (p = 0.002). Severe hypoxia occurred during seven (2.4 %) and 4 (8 %) ATs performed in non-ECMO and ECMO patients (p = 0.063), respectively. Figure S2 (ESM, Additional Results) shows the time course of SpO2 of these 11 severe hypoxic episodes.
Discussion
In this retrospective study, we analyzed the impact of an apnea test strategy based on PEEP application and lung recruitment in a large cohort of patient undergoing BD assessment. Our approach proved to be feasible and safe, even in patients supported by VA-ECMO.
No AT conducted on the patients in this study was aborted, and each AT was completed without clinically relevant complications. There were no instances of cardiac arrhythmias. We did notice a limited increase in vasopressor requirement and need of fluid boluses. Even in patients supported by VA-ECMO, MAP did not change during the AT.
In 3 % of the ATs, we observed short-lasting severe hypoxia at the end of the apnea period. In our institution, we perform two ATs per patient in each BD assessment, which doubles the possibility of occurrence of an hypoxic AT. Indeed, we detected a PaO2 of <40 mmHg during six (3.5 %) of the first ATs and five (2.9 %) of the second ATs. A single patient had severe hypoxia during both of the tests. Severe hypoxia was more frequent in patients having a baseline PaO2/FiO2 <200 mmHg, suggesting the need for careful planning of the AT procedure in this subgroup. Interestingly, severe hypoxia was detected in 2.4 % of ATs performed in non-ECMO patients and in 8 % of ATs performed in ECMO patients. This difference may be explained by the reduced efficiency of membrane lungs in the specific setting of high BF coupled with low GF [23] as well as by differential hypoxia [24]. Although we detected no statistically significant difference in the occurrence of severe hypoxia between ECMO and non-ECMO patients, this counterintuitive result—if confirmed by further studies—should trigger a re-evaluation of the approach to AT in ECMO patients. Notably, despite the occurrence of hypoxia during 3 % of the ATs, no single AT was aborted for hypoxia and—most importantly—no complications, such as arrhythmia or cardiac arrest, were detected. Moreover, the PaO2/FiO2 ratio returned to normal in all of the patients after the ATs. Finally, to further confirm the transiency of the hypoxic condition, we analyzed the SpO2 tracing recorded second by second during the hypoxic AT episodes. The reduction in SpO2 was transient, and the SpO2 level returned to >90 % after just 2 min. Taken together, these observations suggest that our ventilatory management of these patients, including moderate PEEP, low TV coupled with maintenance of PEEP during the apnea phase, and recruitment maneuvers, prevented the occurrence of significant hypoxia and lung de-recruitment.
An analysis of the effects of our approach on organ viability is outside the scope of this study. Nevertheless, albeit we do not have data on post-lung transplant outcomes, we reported high rates of lung harvesting, even in as severely ill population as patients treated with ECMO for cardiac arrest. This high rate may suggest that our approach enhances pulmonary function and thus optimizes the lung viability for subsequent transplantation, as for a “pre-harvesting lung reconditioning”. Further study will be necessary to evaluate this aspect.
A number of relevant methodological differences between this study and previously published series may provide the justification for our positive findings. First, all of the ATs were performed using a PEEP valve. The combination of recruitment maneuvers and PEEP has been suggested to be efficacious in the presence of acute respiratory distress syndrome [25]. In our institution we apply this strategy in all ATs and have achieved positive results; in all cases reported here, even when the patient was hypoxic before the AT, the AT was successfully performed. Second, the ventilatory setup before and during the procedure has been standardized. In this cohort, we adopted a lung protective strategy in which volume-limited ventilation is used in combination with titrated PEEP + a periodic hyperinflation (“sigh”) every 2 min [26]. Third, all of the ATs were performed by a certified intensivist [27].
The impact of AT on oxygenation and hemodynamics of patients undergoing VA-ECMO deserves particular attention. Our study includes the largest consecutive series of ECMO patients undergoing BD determination. VA-ECMO provides both circulatory and gas exchange support. The hemodynamic effects of ECMO depend on the patient residual cardiac function, the use of vasoactive drugs, and the ECMO BF rate. It is thus self-explanatory that reducing the BF is not a possible strategy for BD determination, since it would lead to immediate cardio-circulatory collapse. On the other hand, diminishing the sweep GF may reduce the gas exchange capability of the VA-ECMO support. Nevertheless, a complete abolishment of GF would not only reduce extracorporeal carbon dioxide removal but also oxygen support, and thus lead to severe hypoxia. To avoid these complexities, patient’s on ECMO are usually excluded from BD determination. Indeed, despite reports of BD affecting at least 20 % of ECMO patients (and thus hundreds of patients worldwide), <20 cases of BD determination have been documented to date in this patients’ group [13–16]. This ultimately leads to the loss of a possible large pool of organ donors. In our study, we show the possibility to successfully perform BD determination in ECMO patients by reducing the GF to 1 L/min while providing oxygenation by PEEP application.
Our approach for BD determination in ECMO patients may also be utilized in patients supported by venovenous-ECMO (VV-ECMO). In our experience, BD diagnoses in patients with VV-ECMO are a rarity, since most of these patients die of multi-organ failure [27]. A reasonable assumption to draw is that higher rates of hypoxia would be documented in such a population of patients connected to an ECMO system for respiratory failure.
Because tube clamping was not routinely performed in the ATs reported here, there was a transient loss of PEEP in all patients in the period between disconnection from the ventilator and connection to the resuscitator bag. A ventilator in continuous positive airway pressure (CPAP) mode might be utilized to avoid this loss of PEEP, but this approach has been associated with false triggering and auto-cycling, mainly due to cardiogenic oscillations [28]. On the other hand, opening the duck-bill valve of the resuscitator bag may impose unwarranted inspiratory resistance. Different brands of resuscitator bags may impose different resistances to breathing. Although such resistances have been demonstrated to be clinically meaningless [29], we emphasize the need for continuous monitoring of the patients for eventual inspiratory efforts, since such episodes—regardless of their efficacy—exclude the diagnosis of BD. Assessing the efficacy in guaranteeing oxygenation during AT and discussing the flow dynamics of various resuscitator bags is beyond the scope of this retrospective study. We refer the interested reader to specific works on the topic [29, 30].
We acknowledge several limitations to our study. First, it is retrospective in design. For this reason, some information, such as the frequency of recruitment maneuvers before connection to the resuscitator bag, was not available for analysis. Second, while we reported the OTO score and number of patients selected for lung harvesting, we did not evaluate the post-lung transplant outcomes of our AT technique. Further studies are necessary to evaluate this aspect as well. Moreover, we did not investigate the rate of PaCO2 increase over time in non-ECMO versus ECMO patients during the AT, as information on the timing of disconnection of the patient from mechanical ventilation was not available; future studies should address this issue. Our results are the product of a bundle protocol. Therefore, we cannot assess the single effect of PEEP, of protective ventilation, and of recruitment maneuver on our findings. Last, the average PEEP level used in our population was moderate; therefore, these results may not be relevant to patients with more severe lung disease requiring high levels of positive airway pressure.
In conclusion, the results of this study in a large cohort of consecutive patients, including patients with VA-ECMO, demonstrate that our AT strategy in BD determination based on PEEP application and lung recruitment is both feasible and practical. Future prospective randomized studies comparing our approach for AT with the standard oxygen-diffusion method are warranted.
References
Shemie SD, Hornby L, Baker A et al (2014) International guideline development for the determination of death. Intensive Care Med 40:788–797. doi:10.1007/s00134-014-3242-7
Wijdicks E, Varelas P, Gronseth G, Greer D (2010) Evidence-based guideline update: determining brain death in adults: report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 74:1911–1918. doi:10.1212/WNL.0b013e3181e242a8
Greer DM, Varelas PN, Haque S, Wijdicks EFM (2008) Variability of brain death determination guidelines in leading US neurologic institutions. Neurology 70:284–289. doi:10.1212/01.wnl.0000296278.59487.c2
Citerio G, Crippa IA, Bronco A et al (2014) Variability in brain death determination in Europe: looking for a solution. Neurocrit Care 21:376–382. doi:10.1007/s12028-014-9983-x
Wijdicks EFM, Rabinstein A, Manno EM, Atkinson JD (2008) Pronouncing brain death: contemporary practice and safety of the apnea test. Neurology 71:1240–1244. doi:10.1212/01.wnl.0000327612.69106.4c
Scott JB, Gentile M, Bennett SN et al (2012) Apnea testing during brain death assessment: a review of clinical practice and published literature. Respir Care 58:532–538. doi:10.4187/respcare.01962
Denny JT, Burr A, Tse J et al (2015) A new technique for avoiding barotrauma-induced complications in apnea testing for brain death. J Clin Neurosci 22:1021–1024. doi:10.1016/j.jocn.2014.11.033
Henry NR, Marshall SG (2014) Apnea testing: the effects of insufflation catheter size and flow on pressure and volume in a test lung. Respir Care 59:406–410. doi:10.4187/respcare.02499
Powner DJ (2009) Certification of brain death: take care. Lancet 373:1587–1589. doi:10.1016/S0140-6736(09)60887-4
Melano R, Adum M, Scarlatti A et al (2002) Apnea test in diagnosis of brain death: comparison of two methods and analysis of complications. Transplant Proc 34:11–12. doi:10.1016/S0041-1345(01)02647-1
Goudreau JL, Wijdicks EF, Emery SF (2000) Complications during apnea testing in the determination of brain death: predisposing factors. Neurology 55:1045–1048. doi:10.1212/WNL.56.9.1249
Yee AH, Mandrekar J, Rabinstein A, Wijdicks EFM (2010) Predictors of Apnea test failure during brain death determination. Neurocrit Care 12:352–355. doi:10.1007/s12028-010-9343-4
Jarrah RJ, Ajizian SJ, Agarwal S et al (2014) Developing a standard method for apnea testing in the determination of brain death for patients on venoarterial extracorporeal membrane oxygenation: a pediatric case series. Pediatr Crit Care Med 15:e38–e43. doi:10.1097/PCC.0000000000000006
Shah V, Lazaridis C (2015) Apnea testing on extracorporeal membrane oxygenation: case report and literature review. J Crit Care 30:784–786. doi:10.1016/j.jcrc.2015.03.028
Muralidharan R, Mateen FJ, Shinohara RT et al (2011) The challenges with brain death determination in adult patients on extracorporeal membrane oxygenation. Neurocrit Care 14:423–426. doi:10.1007/s12028-011-9516-9
Smilevitch P, Lonjaret L, Fourcade O, Geeraerts T (2013) Apnea test for brain death determination in a patient on extracorporeal membrane oxygenation. Neurocrit Care 19:215–217. doi:10.1007/s12028-013-9845-y
Goswami S, Evans A, Das B et al (2013) Determination of brain death by apnea test adapted to extracorporeal cardiopulmonary resuscitation. J Cardiothorac Vasc Anesth 27:312–314. doi:10.1053/j.jvca.2012.04.020
Hoskote SS, Fugate JE, Wijdicks EFM (2014) Performance of an apnea test for brain death determination in a patient receiving venoarterial extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth 28:1039–1041. doi:10.1053/j.jvca.2013.12.019
Pirat A, Kömürcü Ö, Yener G, Arslan G (2014) Apnea testing for diagnosing brain death during extracorporeal membrane oxygenation. J Cardiothorac Vasc Anesth 28:e8–e9. doi:10.1053/j.jvca.2013.09.013
Migliaccio ML, Zagli G, Cianchi G et al (2013) Extracorporeal membrane oxygenation in brain-death organ and tissues donors: a single-centre experience. Br J Anaesth 111:673–674. doi:10.1093/bja/aet323
Bruzzone P (2010) Ethical and legal issues in donation after cardiac death in Italy. Transplant Proc 42:1046–1047. doi:10.1016/j.transproceed.2010.03.057
Oto T, Levvey BJ, Whitford H et al (2007) Feasibility and utility of a lung donor score: correlation with early post-transplant outcomes. Ann Thorac Surg 83(1):257–263. doi:10.1016/j.athoracsur.2006.07.040
Lehle K, Philipp A, Hiller KA et al (2014) Efficiency of gas transfer in venovenous extracorporeal membrane oxygenation: analysis of 317 cases with four different ECMO systems. Intensive Care Med 40:1870–1877. doi:10.1007/s00134-014-3489-z
Hou X, Yang X, Du Z et al (2015) Superior vena cava drainage improves upper body oxygenation during veno-arterial extracorporeal membrane oxygenation in sheep. Crit Care 19:68. doi:10.1186/s13054-015-0791-2
Hocker S, Whalen F, Wijdicks EFM (2014) Apnea testing for brain death in severe acute respiratory distress syndrome: a possible solution. Neurocrit Care 20:298–300. doi:10.1007/s12028-013-9932-0
Patroniti N, Foti G, Cortinovis B et al (2002) Sigh improves gas exchange and lung volume in patients with acute respiratory distress syndrome undergoing pressure support ventilation. Anesthesiology 96:788–794. doi:10.1097/00000542-200204000-00004
Datar S, Fugate J, Rabinstein A et al (2014) Completing the Apnea test: decline in complications. Neurocrit Care 21:392–396. doi:10.1007/s12028-014-9958-y
Peek GJ, Mugford M, Tiruvoipati R et al (2009) Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet 374:1351–1363. doi:10.1016/S0140-6736(09)61069-2
Noujeim C, Bouakl I, El-Khatib M, Bou-Khalil P (2013) Ventilator auto-cycling from cardiogenic oscillations: case report and review of literature. Nurs Crit Care 18:222–228. doi:10.1111/nicc.12029
Hess D, Simmons M (1992) An evaluation of the resistance to flow through the patient valves of twelve adult manual resuscitators. Respir Care 37:432–438
Acknowledgments
This study was financially supported by institutional departmental funds.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest with respect to the work reported in this manuscript.
Additional information
M. Giani and V. Scaravilli contributed equally to this work.
Take-home message: An apnea test strategy based on PEEP application and lung recruitment is safe and feasible without significant complications. This technique is applicable during venoarterial ECMO as well.
This paper followed the STROBE guideline for reporting retrospective studies.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Giani, M., Scaravilli, V., Colombo, S.M. et al. Apnea test during brain death assessment in mechanically ventilated and ECMO patients. Intensive Care Med 42, 72–81 (2016). https://doi.org/10.1007/s00134-015-4105-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-015-4105-6