Abstract
Background
Clinical applications of dexmedetomidine (DEX) for neurosurgical procedures have not been adequately investigated. This study aimed to test the use of DEX infusion, alone or as an adjunct to propofol infusion, as compared to propofol infusion in patients with an unruptured cerebral aneurysm after uneventful intracranial procedures.
Methods
In this retrospective observational study from a single institute, of 184 patients who underwent uneventful intracranial procedures for an unruptured cerebral aneurysm between January 2003 and March 2007, we reviewed 50 managed with DEX-based sedation (DEX alone or as an adjunct to propofol infusion) between April 2005 and March 2007, and 50 managed with propofol-based sedation (propofol alone) between January 2003 and April 2005. With DEX-based sedation, both intubated and extubated patients received DEX infusion at an initial dose of 0.4 μg/kg/h, followed by a maintenance dose of 0.2–0.7 μg/kg/h. Propofol was used in both groups at a dose range of 0.5–5.0 mg/kg/h. Hemodynamic variables, including heart rate (HR) and blood pressure (BP), and adverse events were recorded and compared between the groups.
Results
HR during sedation and systolic BP at 2 h after beginning sedation were significantly lower in the DEX group. No serious adverse events were observed. In the DEX group, 66% were sedated in combination with propofol, of whom 94% were intubated.
Conclusions
DEX could be used safely for both intubated and extubated patients following uneventful intracranial procedures for an unruptured cerebral aneurysm, though it significantly reduced HR. Our findings also indicate that it is preferable to add low-dose propofol to DEX for management of intubated patients.
Similar content being viewed by others
Introduction
Dexmedetomidine (DEX) is a highly selective α2-adrenergic agonist that displays sedative, analgesic, anxiolytic, and sympatholytic effects without significant respiratory depression [1–5]. Reductions in levels of post-operative stress response and pain have also been reported to be clinical benefits of α2-adrenergic agonists [1, 6, 7]. In 2004, DEX was approved in Japan for use as a sedative agent in the intensive care unit (ICU) for short-term sedation (<24 h). However, clinical applications of DEX for intracranial procedures have not been adequately reported, though several studies have described its usefulness for awake intraoperative cortical mapping following craniotomy and awake carotid endarterectomy procedures [2, 8–11]. We introduced DEX-based sedation (DEX infusion alone or as an adjunct to propofol infusion) for post-operative management following uneventful intracranial surgery in patients treated for an unruptured cerebral aneurysm. This retrospective review of results from a single institute was performed to investigate the hemodynamic effects and safety of DEX-based sedation as compared to propofol-based sedation (propofol alone infusion). Hemodynamic variables, including heart rate (HR) and blood pressure (BP), were recorded and compared between both groups. The primary outcome measurement was bradycardia during sedation, while the secondary outcome measurements were hypotension and hypertension events. Respiratory adverse events, delayed awakening, and early extubation were also included as other outcomes.
Methods
From January 2003 to March 2007, 184 patients with an unruptured cerebral aneurysm were surgically treated in the Department of Neurosurgery of Higashiosaka City General Hospital. Of those, patients who met the following inclusion criteria were investigated: (1) unruptured cerebral aneurysm located in anterior circulation; (2) uneventful intracranial surgery performed via a pterional approach; (3) total intravenous anesthesia with propofol during the operation; and (4) sedation started in the ICU and continued to the next morning with a minimum duration of 12 h. Patients undergoing a second surgery such as for a bilateral middle cerebral artery aneurysm were excluded, as were patients with a body weight greater than 100 kg, and those with chronic renal failure requiring hemodialysis, with psychiatric disorders, or receiving a psychotropic agent as medication.
Using a mixture of air, oxygen, and 1% propofol (Diprivan®, AstraZeneka, Osaka, Japan), individual anesthesiologists induced general anesthesia, which was maintained with total intravenous anesthesia with propofol. Fentanyl was used as the analgesic agent and controlled ventilation was maintained with intravenous vecuronium during the operation. One surgeon performed neck clipping and/or wrapping with a pterional approach via a frontotemporal craniotomy. After the operation, each patient was transferred to the ICU under intubation and managed with an artificial ventilator in continuous positive airway pressure mode or synchronized intermittent mandatory ventilation in pressure support mode. Muscle relaxants were not given after the operation. Once propofol infusion was discontinued, neurological assessment of each patient was performed, after which they were sedated with propofol or DEX-based sedation again until the next morning, with a minimum duration of 12 h.
From January 2003 to April 2005, propofol was used as the primary sedative agent (propofol-based sedation). Individual anesthesiologists determined the initial infusion rate after considering intraoperative hemodynamics. Dosages ranged from 0.5 to 5.0 mg/kg/h, then were controlled according to the efficiency of sedation and hemodynamic state. From April 2005 to March 2007, DEX was introduced as a sedative agent and used for DEX-based sedation. DEX (Precedex®, Maruishi Pharmaceutical, Osaka, Japan) was infused at an initial dose of 0.4 μg/kg/h without a loading infusion and then adjusted to 0.2–0.7 μg/kg/h. Bolus infusions of DEX were not allowed. If adequate sedation was not obtained with DEX alone, additional propofol was given at an initial dose of 0.5 mg/kg/h and then adjusted up to a maximum of 5.0 mg/kg/h. Patients in the DEX group were allowed to be extubated when clinically indicated, such as with the appearance of agitation, after which DEX administration was continued.
All patients had continuous arterial BP monitoring via a radial artery. Treatments for hemodynamic effects during sedation were conducted according to the following clinically relevant criteria: tachycardia (HR > 100 bpm), bradycardia (HR < 50 bpm), hypertension (systolic BP > 160 mmHg), and hypotension (systolic BP < 90 mmHg). Systolic BP was controlled within 100–160 mmHg. Hypertension was treated with intravenous nicardipine, hypotension with adequate volume loading and/or dopamine administration, tachycardia with control of sedation and an analgesic agent with a diclofenac sodium suppository, and bradycardia with control of DEX, which was discontinued if needed, and atropine administration. A bolus infusion of propofol was allowed for emergency agitation, bucking, and bronchial suctioning in both groups.
We reviewed the patient demographics including age, sex, body weight, and height from the medical records. Pre-operative medical problems including hypertension, cardiovascular problems, and cerebrovascular disease, which may influence hemodynamic variables and neurological states, were also assessed. Data for anesthesia and operation times, and total dosages of propofol and fentanyl were obtained from the anesthesia charts. We reviewed clinically relevant adverse events and the temporal profiles of hemodynamic variables including HR and BP using the ICU charts. Baseline values for HR, and systolic and diastolic BP were set at the beginning of sedation with stable vital signs. These hemodynamic variables were recorded at 2-h intervals from the beginning of sedation to 12 h after beginning sedation and were compared between the groups. Data for total dosages of propofol and DEX, and sedation time were also obtained from the ICU charts. Fluid balance was calculated from infusion and urine volume data obtained from both the anesthesia and ICU charts. The primary outcome of this study was bradycardia during sedation, which was assessed by clinically relevant events and a defined value (HR < 50 bpm and/or atropine required). The secondary outcomes were hypotension (systolic BP < 90 mmHg and dopamine infusion required) and hypertension (systolic BP > 160 mmHg and nicardipine infusion required) events. Respiratory adverse events, delayed awakening, and early extubation were also included as other outcomes. HR and BP values are shown as the mean ± standard deviation (SD). Other data are also presented as the mean ± SD, with the mean values of normally distributed data compared with t-tests. A Mann–Whitney test was applied for nonparametric data, and presented as the median and interquartile ranges. Nominal data were compared using chi-squared analysis. P values less than 0.05 were considered to be statistically significant. All analyses were conducted using SPSS version 18 for Macintosh (SPSS, Inc., Chicago, IL).
Results
One hundred patients who met the inclusion criteria were studied, with 50 in each group. Demographic data and baseline characteristics were comparable between the groups (Table 1). Hypertension was commonly seen in 30 (59%) in the DEX group and in 28 (53%) in the propofol group, while cardiovascular problems, including history of myocardial infarction, angina pectoris, atrial fibrillation, and supraventricular arrhythmia, were found in 5 (10%) in the DEX group and in 10 (20%) in the propofol group. Electrocardiogram abnormalities such as sinus bradycardia and complete or incomplete right bundle branch blocks were also noted, whereas second- or third-degree heart block was not identified in either group. Four (8%) in the DEX group and 7 (13%) in the propofol group had a history of cerebrovascular disease, including cerebral infarction and intracerebral hemorrhage. There were no statistically significant differences in regard to operation time, anesthesia time, total dosage of propofol and fentanyl during anesthesia, and total fluid balance (total infusion volume minus total urine volume from beginning of anesthesia to end of sedation) between the groups. The median (interquartile range) duration of DEX infusion was 17.0 h (range, 16.0–18.2 h), which was significantly longer as compared to propofol infusion in the propofol group (15.5 h; range, 14.4–16.6 h) (P < 0.01).
Clinically relevant adverse events associated with the sedation protocol are shown in Table 2. There were 10 (20%) patients with bradycardia in the DEX group, including 4 treated by atropine and 1 who required discontinuation of DEX infusion, whereas none were detected in the propofol group. There was no evidence of fatal arrhythmia detected in either group. The frequency of hypotension requiring dopamine infusion did not differ significantly, as there were 3 (6%) such patients in the DEX group and 1 (2%) in the propofol group. The number of patients with hypertension requiring nicardipine infusion was also comparable, with 28 (56%) in the DEX group and 26 (52%) in the propofol group. The main reason for the high number of patients requiring nicardipine infusion in both groups was emergent hypertension during neurological assessment after discontinuation of propofol. One patient in the DEX group developed atelectasis, which completely resolved later. Awakening delay was seen in 1 patient in each group, both of whom completely awoke later.
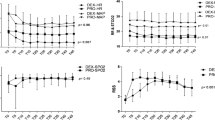
The temporal profiles of HR and systolic and diastolic BP are shown in Figs. 1 and 2. Baseline HR was not significantly different between the groups. However, after beginning sedation, the HR values differed significantly and were slower in the DEX group at all time points (2, 8, 10 h, P < 0.05; 4, 6, 12 h, P < 0.01) (Fig. 1). BP values were comparable between the groups, except for systolic BP at 2 h after sedation, which was significantly lower in the DEX group (P = 0.012) (Fig. 2).
In the DEX group, 33 (66%) required additional propofol (DEX with added propofol subgroup) at a median dosage of 1110.0 mg (interquartile range, 536.0–2365.0 mg), which was more than 50% less as compared to the propofol group, who received a median dosage of 2782.5 mg (interquartile range, 2137.5–3335.0 mg) (P < 0.01) (Fig. 3a). The total dosage of DEX in the DEX with added propofol subgroup (median dosage 432.0 μg, interquartile range 343.0–564.5 μg) was not significantly different from the remaining 17 (34%) patients who were sedated with DEX alone (DEX alone subgroup; median dosage 391.0 μg, interquartile range 306.5–503.5 μg) (P = 0.282) (Fig. 3b). Thirteen (26%) were extubated patients, of whom 11 were sedated with DEX alone and 2 with DEX with added propofol (Fig. 3b, open circles). Consequently, 65% (11 of 17) in the DEX alone subgroup were managed after early extubation, whereas 94% (31 of 33) in the DEX with added propofol subgroup were managed while intubated. None in the propofol group were extubated.
Box and whisker plots of propofol and DEX dosages in the ICU. a The dose of propofol in the DEX with added propofol subgroup was less than 50% as compared to the propofol group (**P < 0.01). b The dose of DEX was not significantly different between the DEX with added propofol and DEX alone subgroups. Closed circle indicates an outlier. Open circles indicate patients with early extubation
Discussion
The present DEX-based sedation was significantly related to reduced HR as compared to propofol-based sedation, while fatal arrhythmia did not occur in any of our patients. Bradycardia is the most typical hemodynamic effect associated with DEX [2, 5–7, 12–16]. In addition to bradycardia, hypertension and hypotension have been reported as adverse events in clinical applications of DEX, especially during loading infusion [1, 6, 7, 13–15, 17]. Various loading infusion rates, such as 1 μg/kg over 10 min [6, 7] and 20 min [17], and 2.5 μg/kg over 10 min [15] have been reported, though 6 μg/kg over 10 min is recommended as the standard loading infusion rate in Japan. After consideration of the potential adverse effects, we did not perform loading infusion of DEX. Aryan [18] et al. also recommend avoiding loading infusion of DEX to manage neurosurgical patients and reported that 3 of 5 patients who received loading infusion experienced hypotension. Although significant hypotension was not commonly observed, systolic BP at 2 h after sedation was significantly lower in the DEX group as compared to the propofol group. In the present study, all patients were awakened once under intubation for neurological assessment, which may have subjected them to stress equivalent to extubation. Therefore, it is possible that in addition to its analgesic sparing effect, DEX shows a hemodynamic stabilizing effect toward stress during the neurological evaluation and up to 2 h later [16].
It remains to be seen whether DEX has effects on the development of vasospasms after cerebral aneurysm clipping. Unlike a ruptured cerebral aneurysm, patients with unruptured cerebral aneurysms after uneventful surgery are less likely to develop vasospasms under the range of hemodynamic changes observed in this study. However, considering the effects of DEX to decrease regional and global cerebral blood flow in human subjects [12, 19], and to reduce cerebral pial vessel diameter in animal studies [20, 21], the drug might cause a conflict to prevent cerebral vasospasms, especially in patients with a subarachnoid hemorrhage.
During management of intubated patients, bronchial suctioning is necessary to prevent pneumonia and atelectasis, which often induce discomfort, agitation, bucking, and emergent hypertension. No serious pulmonary complications were observed in the present DEX group, with only 1 patient reported to have atelectasis. Since a bolus infusion of DEX was not allowed, additional propofol was helpful to solve such problems for intubated patients in the present study. DEX has a sedative effect without significant respiratory depression, thus it can be used for sedation and into the extubation period [22], which explains the significantly longer sedation time in the present DEX group that included both intubated and extubated patients. Agitation, one of the major problems associated with DEX, was observed in 10 of 39 neurosurgical patients in a recent study [18]. In the present study, 65% in the DEX alone subgroup were managed after early extubation, while 94% in the DEX with added propofol subgroup were managed while intubated. These results may reflect a tendency of the attending clinicians to avoid intubated patients becoming agitated in order to obtain easier management.
Results of a recent pilot study suggested that DEX was comparable to propofol or midazolam as a target sedative when used for more than 24 h during mechanical ventilation, though it was not considered suitable for sole deep sedation [23]. We cannot conclude regarding the sedative effect of DEX, as our protocol regarding the introduction of additional propofol was not strict. Even in cases when DEX did not reach the maximum dose, additional propofol was introduced based on the judgment of individual clinicians. Paradoxically, the present results suggest that DEX-based sedation could reduce the dose of propofol by more than 50% as compared to propofol-based sedation. Therefore, DEX may be an optional sedative agent for reducing the dosage of propofol, as high-dose propofol infusion can cause serious adverse events, which have been reported to be components of propofol infusion syndrome [24].
This study has limitations that should be considered. First, the investigated patients were selected from different periods, and studied using both retrospective and observational methods. Second, the sample size was small. Third, strict criteria were not set regarding the introduction of additional propofol or early extubation in the DEX group. In a previous study that employed stricter criteria, including giving propofol after reaching the maximum dose of DEX (0.7 μg/kg/h), only 11% of the patients who underwent DEX-based sedation received additional propofol [17], whereas 66% of the DEX-based sedation patients received additional propofol in the present study. A prospective, randomized, double-blind study is warranted to elucidate the effects of DEX in patients who require neurosurgical management.
In conclusion, DEX-based sedation (DEX alone or as an adjunct to propofol infusion) was safely used for both intubated and extubated patients undergoing uneventful intracranial surgery for an unruptured cerebral aneurysm, and was significantly related to reduced HR during sedation as compared to propofol-based sedation. We consider it preferable to add low-dose propofol to DEX for management of intubated patients. Additional investigation is warranted to elucidate the effectiveness and adverse effects of clinical application of DEX for neurosurgical patients.
References
Kamibayashi T, Maze M. Clinical uses of alpha2-adrenergic agonists. Anesthesiology. 2000;93:1345–9.
Bekker A, Sturaitis MK. Dexmedetomidine for neurological surgery. Neurosurgery. 2005;57:1–10.
Hsu YW, Cortinez LI, Robertson KM, et al. Dexmedetomidine pharmacodynamics. Part I: crossover comparison of the respiratory effects of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1066–76.
Cortinez LI, Hsu YW, Sum-Ping ST, et al. Dexmedetomidine pharmacodynamics. Part II: Crossover comparison of the analgesic effect of dexmedetomidine and remifentanil in healthy volunteers. Anesthesiology. 2004;101:1077–83.
Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Sedative, amnestic, and analgesic properties of small-dose dexmedetomidine infusions. Anesth Analg. 2000;90:699–705.
Arain SR, Ruehlow RM, Uhrich TD, Ebert TJ. The efficacy of dexmedetomidine versus morphine for postoperative analgesia after major inpatient surgery. Anesth Analg. 2004;98:153–8.
Venn RM, Bradshaw CJ, Spencer R, et al. Preliminary UK experience of dexmedetomidine, a novel agent for postoperative sedation in the intensive care unit. Anaesthesia. 1999;54:1136–42.
Bekker AY, Kaufman B, Samir H, Doyle W. The use of dexmedetomidine infusion for awake craniotomy. Anesth Analg. 2001;92:1251–3.
Almeida AN, Tavares C, Tibano A, Sasaki S, Murata KN, Marino R Jr. Dexmedetomidine for awake craniotomy without laryngeal mask. Arq Neuropsiquiatr. 2005;63:748–50.
Ard JL Jr, Bekker AY, Doyle WK. Dexmedetomidine in awake craniotomy: a technical note. Surg Neurol. 2005;63:114–7.
Bekker AY, Basile J, Gold M, et al. Dexmedetomidine for awake carotid endarterectomy: efficacy, hemodynamic profile, and side effects. J Neurosurg Anesthesiol. 2004;16:126–35.
Prielipp RC, Wall MH, Tobin JR, et al. Dexmedetomidine-induced sedation in volunteers decreases regional and global cerebral blood flow. Anesth Analg. 2002;95:1052–9.
Riker RR, Shehabi Y, Bokesch PM, et al. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA. 2009;301:489–99.
Pandharipande PP, Pun BT, Herr DL, et al. Effect of sedation with dexmedetomidine vs lorazepam on acute brain dysfunction in mechanically ventilated patients: the MENDS randomized controlled trial. JAMA. 2007;298:2644–53.
Venn RM, Bryant A, Hall GM, Grounds RM. Effects of dexmedetomidine on adrenocortical function, and the cardiovascular, endocrine and inflammatory responses in post-operative patients needing sedation in the intensive care unit. Br J Anaesth. 2001;86:650–6.
Aho M, Lehtinen AM, Erkola O, Kallio A, Korttila K. The effect of intravenously administered dexmedetomidine on perioperative hemodynamics and isoflurane requirements in patients undergoing abdominal hysterectomy. Anesthesiology. 1991;74:997–1002.
Herr DL, Sum-Ping ST, England M. ICU sedation after coronary artery bypass graft surgery: dexmedetomidine-based versus propofol-based sedation regimens. J Cardiothorac Vasc Anesth. 2003;17:576–84.
Aryan HE, Box KW, Ibrahim D, Desiraju U, Ames CP. Safety and efficacy of dexmedetomidine in neurosurgical patients. Brain Inj. 2006;20:791–8.
Zornow MH, Maze M, Dyck JB, Shafer SL. Dexmedetomidine decreases cerebral blood flow velocity in humans. J Cereb Blood Flow Metab. 1993;13:350–3.
Ohata H, Iida H, Dohi S, Watanabe Y. Intravenous dexmedetomidine inhibits cerebrovascular dilation induced by isoflurane and sevoflurane in dogs. Anesth Analg. 1999;89:370–7.
Ganjoo P, Farber NE, Hudetz A, et al. In vivo effects of dexmedetomidine on laser-Doppler flow and pial arteriolar diameter. Anesthesiology. 1998;88:429–39.
Venn RM, Hell J, Grounds RM. Respiratory effects of dexmedetomidine in the surgical patient requiring intensive care. Crit Care. 2000;4:302–8.
Ruokonen E, Parviainen I, Jakob SM, et al. Dexmedetomidine versus propofol/midazolam for long-term sedation during mechanical ventilation. Intensive Care Med. 2009;35:282–90.
Cremer OL, Moons KG, Bouman EA, Kruijswijk JE, de Smet AM, Kalkman CJ. Long-term propofol infusion and cardiac failure in adult head-injured patients. Lancet. 2001;357:117–8.
Acknowledgments
The authors thank all of the physicians and nursing staff of the ICU, who were actively engaged in the treatment and care of the present patients. This research would not have been accomplished without their cooperation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Yokota, H., Yokoyama, K., Noguchi, H. et al. Post-Operative Dexmedetomidine-Based Sedation After Uneventful Intracranial Surgery for Unruptured Cerebral Aneurysm: Comparison with Propofol-Based Sedation. Neurocrit Care 14, 182–187 (2011). https://doi.org/10.1007/s12028-010-9485-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12028-010-9485-4