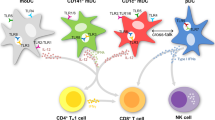
Abstract
Immunotherapy with dendritic cells (DCs), which have been manipulated ex vivo to become immunogenic or tolerogenic, has been tested in clinical trials for disease therapy. DCs are sentinels of the immune system, which after exposure to antigenic or inflammatory signals and crosstalk with effector CD4+ T cells express high levels of costimulatory molecules and cytokines. Upregulation of either costimulatory molecules or cytokines promotes immunologic DCs, whereas their downregulation generates tolerogenic DCs (TDCs), which induce T regulatory cells (Tregs) and a state of tolerance. Immunogenic DCs are used for the therapy of infectious diseases such as HIV-1 and cancer, whereas tolerogenic DCs are used in treating various autoimmune diseases and in transplantation. DC vaccination is still at an early stage, and improvements are mainly needed in quality control of monitoring assays to generate clinical-grade DC products and to assess the effect of DC vaccination in future clinical trials. Here, we review the recent work in DC generation and monitoring approaches for DC-based trials with immunogenic or tolerogenic DCs.

Similar content being viewed by others
References
Langerhans P. Über die Nerven der Menschlichen Haut. Virchows Arch. 1868;44:325–37.
Steinman RM, Cohn ZA. Identification of a novel cell type in peripheral lymphoid organs of mice. I. Morphology, quantitation, tissue distribution. J Exp Med. 1973;137:1142–62.
Steinman RM, Banchereau J. Taking dendritic cells into medicine. Nature. 2007;449:419–27.
Sato K, Fujita S. Dendritic cells: nature and classification. Allergol Int. 2007;56:183–91.
Adorini L, Penna G. Dendritic cell tolerogenicity: a key mechanism in immunomodulation by vitamin D receptor agonists. Hum Immunol. 2009;70:345–52.
Jacobs B, Wuttke M, Papewalis C, Seissler J, Schott M. Dendritic cell subtypes and in vitro generation of dendritic cells. Horm Metab Res. 2008;40:99–107.
Traver D, Akashi K, Manz M, Merad M, Miyamoto T, Engleman EG, Weissman IL. Development of CD8 alpha positive dendritic cells from a common myeloid progenitor. Science. 2000;290:2152–4.
Kapsenberg ML. Dendritic-cell control of pathogen-driven T-cell polarization. Nat Rev Immunol. 2003;3:984–93.
Manicassamy S, Pulendran B. Dendritic cell control of tolerogenic responses. Immunol Rev. 2011;241:206–27.
Mahnke K, Schmitt E, Bonifaz L, Enk AH, Jonuleit H. Immature, but not inactive: the tolerogenic function of immature dendritic cells. Immunol Cell Biol. 2002;80:477–83.
Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–9.
Yamazaki S, Iyoda T, Tarbell K, Olson K, Velinzon K, Inaba K, Steinman RM. Direct expansion of functional CD25+ CD4+ regulatory T cells by antigen-processing dendritic cells. J Exp Med. 2003;198:235–47.
Wu J, Horuzsko A. Expression and function of immunoglobulin-like transcripts on tolerogenic dendritic cells. Hum Immunol. 2009;70:353–6.
Steinman RM, Hawiger D, Nussenzweig MC. Tolerogenic dendritic cells. Annu Rev Immunol. 2003;21:685–711.
Matta BM, Castellaneta A, Thomson AW. Tolerogenic plasmacytoid DC. Eur J Immunol. 2010;40:2667–76.
Connolly NC, Whiteside TL, Wilson C, Kondragunta V, Rinaldo CR, Riddler SA. Therapeutic immunization with HIV-1peptide-loaded dendritic cells is safe and immunogenic in HIV-1-infected individuals. Clin Vaccine Immunol. 2008;15:284–92.
Gilboa E. DC-based cancer vaccines. J Clin Invest. 2007;117:1195–203.
Ezzelarab M, Thomson AW. Tolerogenic dendritic cells and their role in transplantation. Semin Immunol. 2011; July 6 (In press).
Lo J, Clare-Salzler MJ. Dendritic cell subsets and type I diabetes: focus upon DC-based therapy. Autoimmune Rev. 2006;5:419–23.
Jähnisch H, Füssel S, Kiessling A, et al. Dendritic cell-based immunotherapy for prostate cancer. Clin Dev Immunol. 2010;517493.
Aarntzen EH, Figdor CG, Adema GJ, Punt CJ, de Vries IJ. Dendritic cell vaccination and immune monitoring. Cancer Immunol Immunother. 2008;57:1559–68.
Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–33.
Ueno H, Tcherepanova I, Reygrobellet O, Laughner E, Ventura C, Palucka AK, Banchereau J. Dendritic cell subsets generated from CD34+ hematopoietic progenitors can be transfected with mRNA and induce antigen-specific cytotoxic T cell responses Journal of Immunological Methods. 2004;285:171–80.
Mallon DF, Buck A, Reece JC, Crowe SM, Cameron PU. Monocyte-derived dendritic cells as a model for the study of HIV-1 infection: Productive infection and phenotypic changes during culture in human serum. 1999; Immunol Cell Biol. 77:442–50.
Ozkurt ZN, Yegin ZA, Suyani E, Aki SZ, Acar K, Yagci M, Sucak GT. Factors affecting stem cell mobilization for autologous hematopoietic stem cell transplantation. J Clin Apher. 2010;25:280–6.
Chen CH, Wu TC. Experimental vaccine strategies for cancer immunotherapy. J Biomed Sci. 1998;5:231–52.
Luft T, Pang KC, Thomas E, Bradley CJ, Savoia H, Trapani J, Cebon J. Serum-free culture model for studying the differentiation of human dendritic cells from adult CD34+ progenitor cells. Exp Hematol. 1998;26:489–500.
Lim FT, Kanhai HH, Falkenburg JH. Characterization of the human CD34+ hematopoietic progenitor cell compartment during the second trimester of pregnancy. Haematologica. 2005;90:173–9.
Felzmann T, Witt V, Wimmer D, Ressmann G, Wagner D, Paul P, Hüttner K, Fritsch G. Monocyte enrichment from leukapharesis products for the generation of DCs by plastic adherence, or by positive or negative selection. Cytotherapy. 2003;5:391–8.
Weekx SF, Van Bockstaele DR, Plum J, et al. CD34++ CD38− and CD34+ CD38+ human hematopoietic progenitors from fetal liver, cord blood, and adult bone marrow respond differently to hematopoietic cytokines depending on the ontogenic source. Exp Hematol. 1998;26:1034–42.
Tseng SY, Nishimoto KP, Silk KM, et al. Generation of immunogenic dendritic cells from human embryonic stem cells without serum and feeder cells. Regen Med. 2009;4:513–26.
Michiels A, Tuyaerts S, Bonehill A, Heirman C, Corthals J, Thielemans K. Delivery of tumor-antigen-encoding mRNA into dendritic cells for vaccination. Methods Mol Biol. 2008;423:155–63.
Berger TG, Feuerstein B, Strasser E, Hirsch U, Schreiner D, Schuler G. Schuler-Thurner B.Large-scale generation of mature monocyte-derived dendritic cells for clinical application in cell factories. J Immunol Methods. 2002;268:131–40.
Babatz J, Röllig C, Oelschlägel U, Zhao S, Ehninger G, Schmitz M, Bornhäuser M. Large-scale immunomagnetic selection of CD14+ monocytes to generate dendritic cells for cancer immunotherapy: a phase I study. J Hematother Stem Cell Res. 2003;12:515–23.
Romani N, Gruner S, Brang D, et al. Proliferating dendritic cell progenitors in human blood. J Exp Med. 1994;180:83–93.
Cobb A, Roberts LK, Palucka AK, et al. Development of a HIV-1 lipopeptide antigen pulsed therapeutic dendritic cell vaccine.J Immunol Methods. 2011;365:27–7.
Conti L, Gessani S.GM-CSF in the generation of dendritic cells from human blood monocyte precursors: recent advances. Immunobiology. 2008;213(9–10):859–70.
Cardone M, Varano B, Puddu P, Belardelli F, Gessani S. Role of the cytokine environment and cytokine receptor expression on the generation of functionally distinct dendritic cells from human monocytes. Eur J Immunol. 2008;38:750–62.
Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–18.
Zhou LJ, Tedder TF. Human blood dendritic cells selectively express CD83, a member of the immunoglobulin superfamily. J Immunol. 1995;154:3821–35.
Romani N, Reider D, Heuer M, et al. Generation of mature dendritic cells from human blood. An improved method with special regard to clinical applicability. J Immunol Meth. 1996;196:137–51.
O’Doherty U, Steinman RM, Peng M, et al. Dendritic cells freshly isolated from human blood express CD4 and mature into typical immunostimulatory dendritic cells after culture in monocyte-conditioned medium. J Exp Med. 1993;178:1067–76.
Jonuleit H, Kühn U, Müller G, Steinbrink K, et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. Eur J Immunol. 1997;27:3135–42.
Scandella E, Men Y, Gillessen S, Forster R, Groettrup M. Prostaglandin E2 is a key factor for CCR7 surface expression and migration of monocyte-derived dendritic cells. Blood. 2002;100:1354–61.
Morelli AE, Thomson AW. Dendritic cells under the spell of prostaglandins. Trends Immunol. 2003;24:108–11.
Rosenberg SA, Yang JC, Restifo NP. Cancer immunotherapy: moving beyond current vaccines. Nat Med. 2005;10:909–15.
Mailliard RB, Wankowicz-Kalinska A, et al. Alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64:5934–7.
Santini SM, Lapenta C, Logozzi M, Parlato S, Spada M, Di Pucchio T, Belardelli F. Type I interferon as a powerful adjuvant for monocyte-derived dendritic cell development and activity in vitro and in Hu-PBL-SCID mice. J Exp Med. 2000;191:1777–88.
Della Bella S, Nicola S, Riva A, Biasin M, Clerici M. Villa ML Functional repertoire of dendritic cells generated in granulocyte macrophage-colony stimulating factor and interferon-alpha. J Leukoc Biol. 2004;75:106–16.
Lapenta C, Santini SM, Logozzi M, et al. Potent immune response against HIV-1 and protection from virus challenge in hu-PBL-SCID mice immunized with inactivated virus-pulsed dendritic cells generated in the presence of IFN-alpha. J Exp Med. 2003;198:361–7.
Miyazaki A, et al. TNF-alpha drives human CD14+ monocytes to differentiate into CD70+ dendritic cells evoking Th1 and Th17 responses. J Immunol. 2007;179:1449–57.
Mohamadzadeh M, Berard F, Essert G, et al. Interleukin 15 skews monocyte differentiation into dendritic cells with features of Langerhans cells. J Exp Med. 2001;194:1013–20.
Dubsky P, Saito H, Leogier M, Dantin C, Connolly JE, Banchereau J, Palucka AK. IL-15-induced human DC efficiently prime melanoma-specific naïve CD8+ T cells to differentiate into CTL. Eur J Immunol. 2007;37:1678–90.
Morelli AE, Thomson AW. Dendritic cells: regulators of alloimmunity and opportunities for tolerance induction. Immunol Rev. 2003;196:125–46.
Rutella S, Danese S, Leone G. Tolerogenic dendritic cells: cytokine modulation comes of age. Blood. 2006;108:1435–40.
Lutz MB, Suri RM, Niimi M, et al. Immature dendritic cells generated with low doses of GM-CSF in the absence of IL-4 is maturation resistant and prolongs allograft survival in vivo. Eur J Immunol. 2000;30:1813–22.
Steinbrink K, Wölfl M, Jonuleit H, Knop J, Enk AH. Induction of tolerance by IL-10-treated dendritic cells. J Immunol. 1997;159:4772–80.
Lu L, Li W, Zhong C, Qian S, Fung JJ, Thomson AW, Starzl TE. Increased apoptosis of immunoreactive host cells and augmented donor leukocyte chimerism, not sustained inhibition of B7 molecule expression are associated with prolonged cardiac allograft survival in mice preconditioned with immature donor dendritic cells plus anti-CD40L mAb. Transplantation. 1999;68:747–57.
Oyama T, Ran S, Ishida T, Nadaf S, Kerr L, Carbone DP, Gabrilovich DI. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor kappa B activation in hemopoietic progenitor cells. J Immunol. 1998;160:1224–32.
Hackstein H, Thomson AW. Dendritic cells: emerging pharmacological targets of immunosuppressive drugs. Nat Rev Immunol. 2004;4:24–34.
Hackstein H, et al. Rapamycin inhibits IL-4-induced dendritic cell maturation in vitro and dendritic cell mobilization and function in vivo. Blood. 2003;101:4457–63.
Hu J, Wan Y. Tolerogenic dendritic cells and their potential applications. Immunology. 2011;132:307–14.
Taher YA, van Esch BC, Hofman GA, Henricks PA, van Oosterhout AJ. 1 alpha, 25-dihydroxyvitamin D3 potentiates the beneficial effects of allergen immunotherapy in a mouse model of allergic asthma: role for IL-10 and TGF-beta. J Immunol. 2008;180:5211–21.
Crooke ST. Progress in antisense technology. Ann Rev Med. 2004;55:61–95.
Fjose A, Ellingsen S, Wargelius A, Seo HC. RNA interference: mechanisms and applications. Biotechnol Annu Rev. 2001;7:31–57.
Elbashir SM, Harborth J, Lendeckel W, Yalcin A, Weber K, Tuschl T. Duplexes of 21-nucleotide RNAs mediate RNA interference in cultured mammalian cells. Nature. 2001;411:494–8.
Soumelis V, Reche PA, Kanzler H, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–80.
Reche PA, Soumelis V, Gorman DM, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol. 2001;167:336–43.
Rissoan MC, Soumelis V, Kadowaki N, Grouard G, Briere F, de Waal Malefyt R, Liu YJ. Reciprocal control of T helper cell and dendritic cell differentiation. Science. 1999;283:1183–6.
Buelens C, Bartholomé EJ, Amraoui Z, et al. Interleukin-3 and interferon beta cooperate to induce differentiation of monocytes into dendritic cells with potent helper T-cell stimulatory properties. Blood. 2002;99:993–8.
Ebner S, Hofer S, Nguyen VA, et al. A novel role for IL-3: human monocytes cultured in the presence of IL-3 and IL-4 differentiate into dendritic cells that produce less IL-12 and shift Th cell responses toward a Th2 cytokine pattern. J Immunol. 2002;168:6199–207.
Nicolette CA, Healey D, Tcherepanova I, et al. Dendritic cells for active immunotherapy: optimizing design and manufacture in order to develop commercially and clinically viable products. Vaccine. 2007;25:B47–60.
Giordano R, Lazzari L, Rebulla P. Clinical grade cell manipulation. Vox Sang. 2004;87:65–72.
Whiteside TL. Evaluation of dendritic cell products generated for human therapy and post-treatment immune monitoring. Bio Pharm Int. 2008;21: 42–67.
Soncin S, Lo Cicero V, Astori G, Soldati G, Gola M, Sürder D, Moccetti T. A practical approach for the validation of sterility, endotoxin and potency testing of bone marrow mononucleated cells used in cardiac regeneration in compliance with good manufacturing practice. J Transl Med. 2009;7:78.
Meyer-Wentrup F, Burdach S. Efficacy of dendritic cell generation for clinical use: recovery and purity of monocytes and mature dendritic cells after immunomagnetic sorting or adherence selection of CD14+ starting populations. J Hematother Stem Cell Res. 2003;12:289–99.
Ishri RK, Menzies S, Hersey P, Halliday GM. Rapid downregulation of antigen processing enzymes in ex vivo generated human monocyte derived dendritic cells occur endogenously in extended cultures. Immunol Cell Biol. 2004;82:239–46.
Butterfield LH, Gooding W, Whiteside TL. Development of a potency assay for human dendritic cells: IL-12p70 production. J Immunother. 2008;31:89–100.
Shankar G, Fourrier MS, Grevenkamp MA, Lodge PA. Validation of the COSTIM bioassay for dendritic cell potency. J Pharm Biomed Anal. 2004;36:285–94.
Whiteside TL. Immune monitoring of clinical trials with biotherapies. Adv Clin Chem. 2008;45:75–97.
Baban B, Chandler PR, Johnson BA, et al. Physiologic control of IDO competence in splenic dendritic cells. J Immunol. 2011;187:2329–35.
Morelli AE, Thomson AW. Tolerogenic dendritic cells and the quest for transplant tolerance. Nat Rev Immunol. 2007;7:610–21.
Munn DH, Sharma MD, Hou D, et al. Expression of indoleamine 2, 3-dioxygenase by plasmacytoid dendritic cells in tumor-draining lymph nodes. J Clin Invest. 1994;114:280–90.
Mellor AL, Chandler P, Baban B, et al. Specific subsets of murine dendritic cells acquire potent T cell regulatory functions following CTLA4-mediated induction of indoleamine 2, 3 dioxygenase. Int Immunol. 2004;16:1391–401.
Mellor AL, Baban B, Chandler PR, Manlapat A, Kahler DJ, Munn DH. Cutting edge: CpG oligonucleotides induce splenic CD19+ dendritic cells to acquire potent indoleamine 2, 3-dioxygenase-dependent T cell regulatory functions via IFN Type 1 signaling. J Immunol. 2005;175:5601–5.
Mellor AL, Munn DH. Creating immune privilege: active local suppression that benefits friends, but protects foes. Nat Rev Immunol. 2008;8:74–80.
Muller AJ, Sharma MD, Chandler PR, et al. Chronic inflammation that facilitates tumor progression creates localimmune suppression by inducing indoleamine 2, 3 dioxygenase. Proc Natl Acad Sci USA. 2008;105:17073–8.
Sharma MD, et al. Chronic inflammation that facilitates tumor progression creates local nodes activate mature Tregs via indoleamine 2, 3-dioxygenase. J Clin Invest. 2007;17:2570–82.
Sørensen RB, Hadrup SR, Svane IM, Hjortsø MC, Thor Straten P, Andersen MH. Indoleamine 2, 3-dioxygenase specific cytotoxic T cells as immune regulators. Blood. 2011;117:2200–10.
Gurtner GJ, Newberry RD, Schloemann SR, McDonald KG, Stenson WF. Inhibition of indoleamine 2, 3-dioxygenase augments tri nitro benzene sulfonic acid colitis in mice. Gastroenterology. 2003;125:1762–73.
Kwidzinski E, Bunse J, Aktas O, et al. Indolamine 2, 3-dioxygenase is expressed in the CNS and downregulates autoimmune inflammation. FASEB J. 2005;19:1347–9.
Adorini L, Lombardi G,Vasquez YR. Dendritic cells handbook of experimental pharmacology. 2009;188(II):165–190.
Osada T, Clay TM, Woo CY, Morse MA, Lyerly HK. Dendritic cell-based immunotherapy. Int Rev Immunol. 2007;25:377–413.
Acknowledgment
We are very grateful to K. Regan for editing the manuscript.
Conflict of interest
The authors declare no competing financial interests.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kalantari, T., Kamali-Sarvestani, E., Ciric, B. et al. Generation of immunogenic and tolerogenic clinical-grade dendritic cells. Immunol Res 51, 153–160 (2011). https://doi.org/10.1007/s12026-011-8255-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-011-8255-5