Abstract
Purpose
Most differentiated thyroid cancer (DTC) patients have a good prognosis after surgery, but radioiodine refractory differentiated thyroid cancer (RAIR-DTC) patients have a significantly reduced 5-year survival rate (<60%) and a significantly increased recurrence rate (>30%). This study aimed to clarify the tescalcin (TESC) role in promoting the malignant PTC progression and providing a potential target for RAIR-DTC treatment.
Methods
We analyzed TESC expression and clinicopathological characteristics using the Cancer Genome Atlas (TCGA) and performed qRT-PCR on tissue samples. TPC-1 and IHH-4 proliferation, migration, and invasion were detected after transfection with TESC-RNAi. Using Western blot (WB), several EMT-related indicators were detected. Moreover, iodine uptake of TPC-1 and IHH-4 after transfection with TESC-RNAi was detected. Finally, NIS, ERK1/2, and p-ERK1/2 levels were determined by WB.
Results
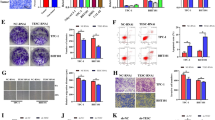
TESC was significantly upregulated in DTC tissues and positively correlated with BRAF V600E mutation based on data analysis from TCGA and our center. Reduced expression of TESC in both IHH-4 (BRAF V600E mutation) and TPC-1 (BRAF V600E wild type) cells significantly inhibited cell proliferation, migration, and invasion. It downregulated the EMT pathway markers Vimentin and N-cadherin, and increased E- cadherin. Moreover, TESC knockdown significantly inhibited ERK1/2 phosphorylation and decreased NIS expression in DTC cells, with a remarkably increased iodine uptake rate.
Conclusions
TESC was highly expressed in DTC tissues and may have promoted metastasis through EMT and induced iodine resistance by downregulating NIS in DTC cells.



Similar content being viewed by others
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
K.D. Miller, L. Nogueira, T. Devasia, A.B. Mariotto, K.R. Yabroff, A. Jemal, et al. Cancer treatment and survivorship statistics, 2022. CA Cancer J. Clin. 72(5), 409–436 (2022).
R.L. Siegel, K.D. Miller, H.E. Fuchs, A. Jemal, Cancer statistics, 2022. CA Cancer J. Clin. 72(1), 7–33 (2022)
L.G. Morris, A.R. Shaha, R.M. Tuttle, A.G. Sikora, I. Ganly, Tall-cell variant of papillary thyroid carcinoma: a matched-pair analysis of survival. Thyroid 20(2), 153–8 (2010)
R.I. Haddad, C. Nasr, L. Bischoff, N.L. Busaidy, D. Byrd, G. Callender et al. NCCN guidelines insights: thyroid carcinoma, version 2.2018. J. Natl Compr. Canc Netw. 16(12), 1429–40 (2018)
Y. Lin, S. Qin, Z. Li, H. Yang, W. Fu, S. Li et al. Apatinib vs placebo in patients with locally advanced or metastatic, radioactive iodine-refractory differentiated thyroid cancer: the REALITY randomized clinical trial. JAMA Oncol. 8(2), 242–50 (2022)
K.G. Kolobynina, V.V. Solovyova, K. Levay, A.A. Rizvanov, V.Z. Slepak, Emerging roles of the single EF-hand Ca2+ sensor tescalcin in the regulation of gene expression, cell growth and differentiation. J. Cell Sci. 129(19), 3533–40 (2016)
K. Levay, V.Z. Slepak, Tescalcin is an essential factor in megakaryocytic differentiation associated with Ets family gene expression. J. Clin. Investig. 117(9), 2672–83 (2007)
J.H. Lee, S.I. Choi, R.K. Kim, E.W. Cho, I.G. Kim, Tescalcin/c-Src/IGF1Rβ-mediated STAT3 activation enhances cancer stemness and radioresistant properties through ALDH1. Sci. Rep. 8(1), 10711 (2018)
Z.G. Zhou, J.B. Chen, R.X. Zhang, L. Ye, J.C. Wang, Y.X. Pan et al. Tescalcin is an unfavorable prognosis factor that regulats cell proliferation and survival in hepatocellular carcinoma patients. Cancer Commun. 40(8), 355–69 (2020)
Y.H. Kang, S.R. Han, J.T. Kim, S.J. Lee, Y.I. Yeom, J.K. Min et al. The EF-hand calcium-binding protein tescalcin is a potential oncotarget in colorectal cancer. Oncotarget 5(8), 2149–60 (2014)
T.W. Kim, S.R. Han, J.T. Kim, S.M. Yoo, M.S. Lee, S.H. Lee et al. Differential expression of tescalcin by modification of promoter methylation controls cell survival in gastric cancer cells. Oncol. Rep. 41(6), 3464–74 (2019)
X. Zou, Q. Zhou, Y. Nie, J. Gou, J. Yang, J. Zhu et al. Tescalcin promotes highly invasive papillary thyroid microcarcinoma by regulating FOS/ERK signaling pathway. BMC Cancer 22(1), 595 (2022)
Y. Fan, X. Fan, H. Yan, Z. Liu, X. Wang, Q. Yuan et al. Long non-coding ROR promotes the progression of papillary thyroid carcinoma through regulation of the TESC/ALDH1A1/TUBB3/PTEN axis. Cell Death Dis. 13(2), 157 (2022)
L. Stein, J. Rothschild, J. Luce, J.K. Cowell, G. Thomas, T.I. Bogdanova et al. Copy number and gene expression alterations in radiation-induced papillary thyroid carcinoma from chernobyl pediatric patients. Thyroid 20(5), 475–87 (2010)
M. D’Agostino, M. Sponziello, C. Puppin, M. Celano, V. Maggisano, F. Baldan et al. Different expression of TSH receptor and NIS genes in thyroid cancer: role of epigenetics. J. Mol. Endocrinol. 52(2), 121–31 (2014)
I.L. Wapnir, M. van de Rijn, K. Nowels, P.S. Amenta, K. Walton, K. Montgomery et al. Immunohistochemical profile of the sodium/iodide symporter in thyroid, breast, and other carcinomas using high density tissue microarrays and conventional sections. J. Clin. Endocrinol. Metab. 88(4), 1880–8 (2003)
American Thyroid Association Guidelines Taskforce on Thyroid N, Differentiated Thyroid C, D.S. Cooper, G.M. Doherty, B.R. Haugen, R.T. Kloos et al. Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19(11), 1167–214 (2009)
T.H. Kim, Y.J. Park, J.A. Lim, H.Y. Ahn, E.K. Lee, Y.J. Lee et al. The association of the BRAF(V600E) mutation with prognostic factors and poor clinical outcome in papillary thyroid cancer: a meta-analysis. Cancer 118(7), 1764–73 (2012)
J.M. Oh, B.C. Ahn, Molecular mechanisms of radioactive iodine refractoriness in differentiated thyroid cancer: Impaired sodium iodide symporter (NIS) expression owing to altered signaling pathway activity and intracellular localization of NIS. Theranostics 11(13), 6251–77 (2021)
G. Riesco-Eizaguirre, P. Gutiérrez-Martínez, M.A. García-Cabezas, M. Nistal, P. Santisteban, The oncogene BRAF V600E is associated with a high risk of recurrence and less differentiated papillary thyroid carcinoma due to the impairment of Na+/I- targeting to the membrane. Endocr. Relat. Cancer 13(1), 257–69 (2006)
D. Liu, Z. Liu, S. Condouris, M. Xing, BRAF V600E maintains proliferation, transformation, and tumorigenicity of BRAF-mutant papillary thyroid cancer cells. J. Clin. Endocrinol. Metab. 92(6), 2264–71 (2007)
H. Ouyang, X. He, G. Li, H. Xu, X. Jia, Q. Nie et al. Deep sequencing analysis of miRNA expression in breast muscle of fast-growing and slow-growing broilers. Int. J. Mol. Sci. 16(7), 16242–62 (2015)
M. Faria, D. Félix, R. Domingues, M.J. Bugalho, P. Matos, A.L. Silva, Antagonistic effects of RAC1 and tumor-related RAC1b on NIS expression in thyroid. J. Mol. Endocrinol. 63(4), 309–20 (2019)
M. Xing, BRAF mutation in papillary thyroid cancer: pathogenic role, molecular bases, and clinical implications. Endocr. Rev. 28(7), 742–62 (2007)
I. Karnik, R. Sutherland, J. Elson, S. Aspinall, A. Meeson, TGF-β, to target or not to target; to prevent thyroid cancer progression? Biochim. Biophys. Acta Rev. Cancer 1877(4), 188752 (2022)
B. Mitchell, J.K. Dhingra, M. Mahalingam, BRAF and epithelial-mesenchymal transition: lessons from papillary thyroid carcinoma and primary cutaneous melanoma. Adv. Anat. Pathol. 23(4), 244–71 (2016)
H. Shakib, S. Rajabi, M.H. Dehghan, F.J. Mashayekhi, N. Safari-Alighiarloo, M. Hedayati, Epithelial-to-mesenchymal transition in thyroid cancer: a comprehensive review. Endocrine 66(3), 435–55 (2019)
D. Chakravarty, E. Santos, M. Ryder, J.A. Knauf, X.H. Liao, B.L. West et al. Small-molecule MAPK inhibitors restore radioiodine incorporation in mouse thyroid cancers with conditional BRAF activation. J. Clin. Invest. 121(12), 4700–11 (2011)
S.M. Rothenberg, D.G. McFadden, E.L. Palmer, G.H. Daniels, L.J. Wirth, Redifferentiation of iodine-refractory BRAF V600E-mutant metastatic papillary thyroid cancer with dabrafenib. Clin. Cancer Res. 21(5), 1028–35 (2015)
Funding
This work was supported by grants from the National Natural Science Foundation of China (82103199; 22177104); Natural Science Foundation of Zhejiang Province (LY23H160024); Medical Health Science and Technology Project of Zhejiang Provincial Health Commission (2021KY482, 2023RC001); Traditional Chinese Medicine Science and Technology Project of Zhejiang Provincial Health Commission (2023ZL240); Zhejiang Province Postdoctoral Research Excellence Funding Project (ZJ2021167); The incubation project of the First Affiliated Hospital of Wenzhou Medical University (FHY2019003). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.G. and G.Z. conceived and designed the experiments; Y.G. and Y.C. performed the searching and collected the data; F.S., L.Z., Y.H., Y.L., W.M., Q.Z., L.D., L.L. performed the experiment and analyzed the data; Y.G., J.G. interpretated that results and drafted the manuscript. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethics approval and consent to participate
This study was approved by the Ethical Committee of Zhejiang Provincial People’s Hospital (Approval No. 2019KY298), Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guo, Y., Cai, Y., Song, F. et al. TESC promotes differentiated thyroid cancer development by activating ERK and weakening NIS and radioiodine uptake. Endocrine 81, 503–512 (2023). https://doi.org/10.1007/s12020-023-03350-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-023-03350-6