Abstract
Background
Hyperglycaemia has been indicated as a pro-tumoural factor; however, the prognostic role of diabetes mellitus (DM) in pancreatic neuroendocrine tumours (panNETs) remains ambiguous, partly due to the effects of anti-diabetic drugs. We hypothesise that the blood sugar level per se affects the outcome of panNETs, and thus, we investigated the prognostic significance of the fasting blood glucose (FBG) level in resected panNET patients with no pre-existing DM.
Methods
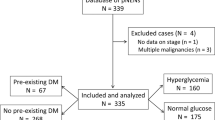
A retrospective cohort study comprising 201 patients with radically resected non-functional panNETs was conducted. A total of 164 patients without pre-existing DM were further studied. An FBG level greater than 5.6 mmol/L was defined as high (otherwise, normal). Survival was evaluated using Kaplan–Meier methods and log-rank tests. Multivariate analyses for survival were performed using the Cox regression model.
Results
High FBG levels were significantly associated with poor overall survival (OS; p = 0.019) and recurrence-free survival (RFS; p = 0.011) in resected patients with panNET who had no pre-existing DM. The multivariable-adjusted hazard ratios (HRs) for mortality and recurrence comparing patients with high and normal FBG levels were 12.19 (95% confidence interval (CI) = 1.15–128.78, p = 0.038) and 2.43 (95% CI = 1.03–5.72, p = 0.042), respectively.
Conclusion
A pre-operative FBG level greater than 5.6 mmol/L is associated with poor OS and RFS metastasis for patients with panNET who undergo radical surgical resection.



Similar content being viewed by others
References
M. Cives, J.R. Strosberg, Gastroenteropancreatic neuroendocrine tumours. CA Cancer J Clin. 68(6), 471–487 (2018). https://doi.org/10.3322/caac.21493
T.R. Halfdanarson, J. Rubin, M.B. Farnell, C.S. Grant, G.M. Petersen, Pancreatic endocrine neoplasms: epidemiology and prognosis of pancreatic endocrine tumours. Endocr. Relat. Cancer 15(2), 409–427 (2008). https://doi.org/10.1677/ERC-07-0221
F. Inzani, G. Petrone, G. Rindi, The new World Health Organization classification for pancreatic neuroendocrine neoplasia. Endocrinol. Metab. Clin. 47(3), 463–470 (2018)
A.D. Singhi, D.S. Klimstra, Well-differentiated pancreatic neuroendocrine tumours (PanNETs) and poorly differentiated pancreatic neuroendocrine carcinomas (PanNECs): concepts, issues and a practical diagnostic approach to high-grade (G3) cases. Histopathology 72(1), 168–177 (2018). https://doi.org/10.1111/his.13408
J. Franko, W. Feng, L. Yip, E. Genovese, A.J. Moser, Non-functional neuroendocrine carcinoma of the pancreas: Incidence, tumour biology, and outcomes in 2,158 patients. J. Gastrointest. Surg. 14(3), 541–548 (2010). https://doi.org/10.1007/s11605-009-1115-0
J. Wu et al. Non-functional pancreatic neuroendocrine tumours: emerging trends in incidence and mortality. BMC Cancer 19(1), 334 (2019)
I. Madeira et al. Prognostic factors in patients with endocrine tumours of the duodenopancreatic area. Gut. 43(3), 422–427 (1998). https://doi.org/10.1136/gut.43.3.422
R. Bettini et al. Prognostic factors at diagnosis and value of WHO classification in a mono-institutional series of 180 non-functioning pancreatic endocrine tumours. Ann. Oncol. 19(5), 903–908 (2008). https://doi.org/10.1093/annonc/mdm552
M. Gallo, R.M. Ruggeri, G. Muscogiuri, G. Pizza, A. Faggiano, A. Colao, Diabetes and pancreatic neuroendocrine tumours: which interplays, if any? Cancer Treat. Rev. 67(December 2017), 1–9 (2018). https://doi.org/10.1016/j.ctrv.2018.04.013
W. Li et al. Effects of hyperglycemia on the progression of tumour diseases. J. Exp. Clin. Cancer Res. 38(1), 1–7 (2019). https://doi.org/10.1186/s13046-019-1309-6
Q. Ben et al. Risk factors for sporadic pancreatic neuroendocrine tumours: a case-control study. Sci. Rep. 6(1), 36073 (2016). https://doi.org/10.1038/srep36073
T.R. Halfdanarson et al. Risk factors for pancreatic neuroendocrine tumours: a clinic-based case-control study. Pancreas 43(8), 1219–1222 (2014). https://doi.org/10.1097/MPA.0000000000000234
G. Capurso et al. Risk factors for sporadic pancreatic endocrine tumours: a case-control study of prospectively evaluated patients. Am. J. Gastroenterol. 104(12), 3034–3041 (2009)
S. Pusceddu et al. Metformin use is associated with longer progression-free survival of patients with diabetes and pancreatic neuroendocrine tumours receiving everolimus and/or somatostatin analogues. Gastroenterology 155(2), 479–489 (2018)
A.D. Association, 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2020. Diabetes Care 43(Supplement 1), S14–S31 (2020)
I.D. Nagtegaal et al. The 2019 WHO classification of tumours of the digestive system. Histopathology 76(2), 182–188 (2020)
E.A. Eisenhauer, P. Therasse, J. Bogaerts et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur. J. Cancer 45(2), 228–247 (2009)
N.H. Cho et al. IDF diabetes Atlas: global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 138, 271–281 (2018). https://doi.org/10.1016/j.diabres.2018.02.023
T.Y. Ryu, J. Park, P.E. Scherer, Hyperglycemia as a risk factor for cancer progression. Diabetes Metab. J. 38(5), 330–336 (2014). https://doi.org/10.4093/dmj.2014.38.5.330
L. de Mestier et al. The postoperative occurrence or worsening of diabetes mellitus may increase the risk of recurrence in resected pancreatic neuroendocrine tumours. Neuroendocrinology (2019). Epub ahead of print
M. Sandini et al. Pre-operative dysglycemia is associated with decreased survival in patients with pancreatic neuroendocrine neoplasms. Surgery 167(3), 575–580 (2020). https://doi.org/10.1016/j.surg.2019.11.007
A. Sharma, T.C. Smyrk, M.J. Levy, M.A. Topazian, S.T. Chari, Fasting blood glucose levels provide estimate of duration and progression of pancreatic cancer before diagnosis. Gastroenterology 155(2), 490–500 (2018)
M. Sandini et al. Pre-operative dysglycemia is associated with decreased survival in patients with pancreatic neuroendocrine neoplasms. Surgery 167(3), 575–580 (2020)
Funding
This work was supported by the National Natural Science Foundation of China (grant numbers 81625016, 81871940, 81902417), the Scientific Innovation Project of Shanghai Education Committee (2019-01-07-00-07-E00057), Clinical and Scientific Innovation Project of Shanghai Hospital Development Centre (SHDC12018109), the Shanghai Natural Science Foundation (grant number 17ZR1406300), the Shanghai Cancer Centre Foundation for Distinguished Young Scholars (grant number YJJQ201803), and the Fudan University Personalised Project for “Double Top” Original Research (grant number XM03190633).
Author contributions
All authors contributed to the study conception and design. C.L. and X.Y.: conceptualisation and funding acquisition. P.Z., Z.F., K.J. and H.C.: resources and investigation and data curation. S.D., Q.H. and Y.Q.: project administration. Q.N. and G.L.: review and editing. Y.G. and Z.F.: formal analysis and software and writing—original draft. All authors read and approved the final paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Ethical approval was waived by the Ethics Board of Shanghai Cancer Centre, Fudan University in view of the retrospective nature of the study and all the procedures being performed were part of the routine care.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Gong, Y., Fan, Z., Zhang, P. et al. High pre-operative fasting blood glucose levels predict a poor prognosis in patients with pancreatic neuroendocrine tumour. Endocrine 71, 494–501 (2021). https://doi.org/10.1007/s12020-020-02469-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-020-02469-0