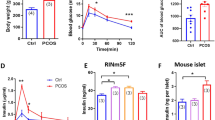
Abstract
Polycystic ovary syndrome (PCOS) patients have intra-ovarian hyperandrogenism and granulosa cells (GCs) from PCOS patients have impaired insulin-dependent glucose metabolism and insulin resistance. The purpose of this study is to determine whether excess androgen affects glucose metabolism and induces insulin resistance of GCs. We firstly explored the insulin metabolic signaling pathway and glucose metabolism in cultured GCs. The Akt phosphorylation and lactate production were increased after insulin treatment. Pre-treatment with PI3-K inhibitor attenuated insulin-induced phosphorylation of Akt and lactate accumulation. However, after treating GCs with different concentrations of testosterone for 5 days, insulin-induced phosphorylation of Akt and lactate production showed no significant change comparing with those of control cells. Finally, mRNA expression of insulin signaling mediators including INSR, IRS-1, IRS-2, and GLUT-4 in GCs was also not significantly altered after testosterone treatment. In conclusion, insulin activates PI3-K/Akt signaling pathway and promotes lactate production in ovarian GCs, but high androgen exerted no obvious influence on insulin signaling pathway and metabolic effect in GCs, suggesting that metabolic actions of insulin in ovarian GCs were unaffected by hyperandrogenism directly.





Similar content being viewed by others
References
M.O. Goodarzi, D.A. Dumesic, G. Chazenbalk, R. Azziz, Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat. Rev. Endocrinol. 7(4), 219–231 (2011)
R.S. Legro, S.A. Arslanian, D.A. Ehrmann, K.M. Hoeger, M.H. Murad, R. Pasquali, C.K. Welt, Diagnosis and treatment of polycystic ovary syndrome: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metab. 98(12), 4565–4592 (2013)
Z.J. Chen, H. Zhao, L. He, Y. Shi, Y. Qin, Z. Li, L. You, J. Zhao, J. Liu, X. Liang, X. Zhao, Y. Sun, B. Zhang, H. Jiang, D. Zhao, Y. Bian, X. Gao, L. Geng, Y. Li, D. Zhu, X. Sun, J.E. Xu, C. Hao, C.E. Ren, Y. Zhang, S. Chen, W. Zhang, A. Yang, J. Yan, J. Ma, Y. Zhao, Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat. Genet. 43(1), 55–59 (2011)
Y. Shi, H. Zhao, Y. Cao, D. Yang, Z. Li, B. Zhang, X. Liang, T. Li, J. Chen, J. Shen, J. Zhao, L. You, X. Gao, D. Zhu, X. Zhao, Y. Yan, Y. Qin, W. Li, J. Yan, Q. Wang, L. Geng, J. Ma, Y. Zhao, G. He, A. Zhang, S. Zou, A. Yang, J. Liu, B. Li, C. Wan, J. Shi, J. Yang, H. Jiang, J.E. Xu, X. Qi, Y. Sun, Y. Zhang, C. Hao, X. Ju, D. Zhao, C.E. Ren, X. Li, W. Zhang, J. Zhang, D. Wu, C. Zhang, L. He, Z.J. Chen, Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat. Genet. 44(9), 1020–1025 (2012)
S. Jonard, D. Dewailly, The follicular excess in polycystic ovaries, due to intra-ovarian hyperandrogenism, may be the main culprit for the follicular arrest. Hum. Reprod. Update 10(2), 107–117 (2004)
K.A. Walters, C.M. Allan, D.J. Handelsman, Rodent models for human polycystic ovary syndrome. Biol. Reprod. 86(5), 149, 1–12 (2012)
C.M. DeUgarte, A.A. Bartolucci, R. Azziz, Prevalence of insulin resistance in the polycystic ovary syndrome using the homeostasis model assessment. Fertil. Steril. 83(5), 1454–1460 (2005)
R. Mathur, C.J. Alexander, J. Yano, B. Trivax, R. Azziz, Use of metformin in polycystic ovary syndrome. Am. J. Obstet. Gynecol. 199(6), 596–609 (2008)
E. Diamanti-Kandarakis, A. Dunaif, Insulin resistance and the polycystic ovary syndrome revisited: an update on mechanisms and implications. Endocr. Rev. 33(6), 981–1030 (2012)
L. Poretsky, N.A. Cataldo, Z. Rosenwaks, L.C. Giudice, The insulin-related ovarian regulatory system in health and disease. Endocr. Rev. 20(4), 535–582 (1999)
Y. Lin, M. Fridstrom, T. Hillensjo, Insulin stimulation of lactate accumulation in isolated human granulosa-luteal cells: a comparison between normal and polycystic ovaries. Hum. Reprod. 12(11), 2469–2472 (1997)
P. Fedorcsak, R. Storeng, P.O. Dale, T. Tanbo, T. Abyholm, Impaired insulin action on granulosa-lutein cells in women with polycystic ovary syndrome and insulin resistance. Gynecol. Endocrinol. 14(5), 327–336 (2000)
X.K. Wu, S.Y. Zhou, J.X. Liu, P. Pollanen, K. Sallinen, M. Makinen, R. Erkkola, Selective ovary resistance to insulin signaling in women with polycystic ovary syndrome. Fertil. Steril. 80(4), 954–965 (2003)
S. Rice, N. Christoforidis, C. Gadd, D. Nikolaou, L. Seyani, A. Donaldson, R. Margara, K. Hardy, S. Franks, Impaired insulin-dependent glucose metabolism in granulosa-lutein cells from anovulatory women with polycystic ovaries. Hum. Reprod. 20(2), 373–381 (2005)
M.C. Richardson, S. Ingamells, C.D. Simonis, I.T. Cameron, R. Sreekumar, A. Vijendren, L. Sellahewa, S. Coakley, C.D. Byrne, Stimulation of lactate production in human granulosa cells by metformin and potential involvement of adenosine 5′ monophosphate-activated protein kinase. J. Clin. Endocrinol. Metab. 94(2), 670–677 (2009)
J.M. Pauli, N. Raja-Khan, X. Wu, R.S. Legro, Current perspectives of insulin resistance and polycystic ovary syndrome. Diabet. Med. 28(12), 1445–1454 (2011)
A. Dunaif, Insulin resistance and the polycystic ovary syndrome: mechanism and implications for pathogenesis. Endocr. Rev. 18(6), 774–800 (1997)
D.A. Dumesic, D.H. Abbott, V. Padmanabhan, Polycystic ovary syndrome and its developmental origins. Rev. Endocr. Metab. Disord. 8(2), 127–141 (2007)
A. Corbould, Chronic testosterone treatment induces selective insulin resistance in subcutaneous adipocytes of women. J. Endocrinol. 192(3), 585–594 (2007)
M.C. Allemand, B.A. Irving, Y.W. Asmann, K.A. Klaus, L. Tatpati, C.C. Coddington, K.S. Nair, Effect of testosterone on insulin stimulated IRS1 Ser phosphorylation in primary rat myotubes—a potential model for PCOS-related insulin resistance. PLoS One 4(1), e4274 (2009)
L. Zhang, Q. Liao, Effects of testosterone and metformin on glucose metabolism in endometrium. Fertil. Steril. 93(7), 2295–2298 (2010)
P. Ormazabal, C. Romero, A.F. Quest, M. Vega, Testosterone modulates the expression of molecules linked to insulin action and glucose uptake in endometrial cells. Horm. Metab. Res. 45(9), 640–645 (2013)
C.P. Zhang, J.L. Yang, J. Zhang, L. Li, L. Huang, S.Y. Ji, Z.Y. Hu, F. Gao, Y.X. Liu, Notch signaling is involved in ovarian follicle development by regulating granulosa cell proliferation. Endocrinology 152(6), 2437–2447 (2011)
K.J. Livak, T.D. Schmittgen, Analysis of relative gene expression data using real-time quantitative PCR and the 2(T)(−Delta Delta C) method. Methods 25(4), 402–408 (2001)
E. Diamanti-Kandarakis, A.G. Papavassiliou, Molecular mechanisms of insulin resistance in polycystic ovary syndrome. Trends Mol. Med. 12(7), 324–332 (2006)
C.M. Taniguchi, B. Emanuelli, C.R. Kahn, Critical nodes in signalling pathways: insights into insulin action. Nat. Rev. Mol. Cell Biol. 7(2), 85–96 (2006)
J.W. Kornfeld, C. Baitzel, A.C. Konner, H.T. Nicholls, M.C. Vogt, K. Herrmanns, L. Scheja, C. Haumaitre, A.M. Wolf, U. Knippschild, J. Seibler, S. Cereghini, J. Heeren, M. Stoffel, J.C. Bruning, Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature 494(7435), 111–115 (2013)
S. Rice, L.J. Pellatt, S.J. Bryan, S.A. Whitehead, H.D. Mason, Action of metformin on the insulin-signaling pathway and on glucose transport in human granulosa cells. J. Clin. Endocrinol. Metab. 96(3), E427–E435 (2011)
K. Hojlund, D. Glintborg, N.R. Andersen, J.B. Birk, J.T. Treebak, C. Frosig, H. Beck-Nielsen, J.F. Wojtaszewski, Impaired insulin-stimulated phosphorylation of Akt and AS160 in skeletal muscle of women with polycystic ovary syndrome is reversed by pioglitazone treatment. Diabetes 57(2), 357–366 (2008)
P. Cetica, L. Pintos, G. Dalvit, M. Beconi, Activity of key enzymes involved in glucose and triglyceride catabolism during bovine oocyte maturation in vitro. Reproduction 124(5), 675–681 (2002)
K. Vendola, J. Zhou, J. Wang, C.A. Bondy, Androgens promote insulin-like growth factor-I and insulin-like growth factor-I receptor gene expression in the primate ovary. Hum. Reprod. 14(9), 2328–2332 (1999)
A. Virkamaki, K. Ueki, C.R. Kahn, Protein–protein interaction in insulin signaling and the molecular mechanisms of insulin resistance. J. Clin. Invest. 103(7), 931–943 (1999)
D.J. Burks, J. Font de Mora, M. Schubert, D.J. Withers, M.G. Myers, H.H. Towery, S.L. Altamuro, C.L. Flint, M.F. White, IRS-2 pathways integrate female reproduction and energy homeostasis. Nature 407(6802), 377–382 (2000)
I. Neganova, H. Al-Qassab, H. Heffron, C. Selman, A.I. Choudhury, S.J. Lingard, I. Diakonov, M. Patterson, M. Ghatei, S.R. Bloom, S. Franks, I. Huhtaniemi, K. Hardy, D.J. Withers, Role of central nervous system and ovarian insulin receptor substrate 2 signaling in female reproductive function in the mouse. Biol. Reprod. 76(6), 1045–1053 (2007)
R.L. Robker, L.K. Akison, B.D. Bennett, P.N. Thrupp, L.R. Chura, D.L. Russell, M. Lane, R.J. Norman, Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J. Clin. Endocrinol. Metab. 94(5), 1533–1540 (2009)
Y.H. Chen, S. Heneidi, J.M. Lee, L.C. Layman, D.W. Stepp, G.M. Gamboa, B.S. Chen, G. Chazenbalk, R. Azziz, miRNA-93 inhibits GLUT4 and is overexpressed in adipose tissue of polycystic ovary syndrome patients and women with insulin resistance. Diabetes 62(7), 2278–2286 (2013)
R. Mioni, S. Chiarelli, N. Xamin, L. Zuliani, M. Granzotto, B. Mozzanega, P. Maffei, C. Martini, S. Blandamura, N. Sicolo, R. Vettor, Evidence for the presence of glucose transporter 4 in the endometrium and its regulation in polycystic ovary syndrome patients. J. Clin. Endocrinol. Metab. 89(8), 4089–4096 (2004)
F. Qu, F.F. Wang, X.E. Lu, M.Y. Dong, J.Z. Sheng, P.P. Lv, G.L. Ding, B.W. Shi, D. Zhang, H.F. Huang, Altered aquaporin expression in women with polycystic ovary syndrome: hyperandrogenism in follicular fluid inhibits aquaporin-9 in granulosa cells through the phosphatidylinositol 3-kinase pathway. Hum. Reprod. 25(6), 1441–1450 (2010)
F. Qu, F.F. Wang, R. Yin, G.L. Ding, M. El-Prince, Q. Gao, B.W. Shi, H.H. Pan, Y.T. Huang, M. Jin, P.C. Leung, J.Z. Sheng, H.F. Huang, A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: hyperandrogenism induces epigenetic alterations in the granulosa cells. J. Mol. Med. (Berl) 90(8), 911–923 (2012)
A. Sen, H. Prizant, A. Light, A. Biswas, E. Hayes, H.J. Lee, D. Barad, N. Gleicher, S.R. Hammes, Androgens regulate ovarian follicular development by increasing follicle stimulating hormone receptor and microRNA-125b expression. Proc. Natl. Acad. Sci. USA 111(8), 3008–3013 (2014)
Acknowledgments
This work was supported by the National Basic Research Program of China (973 program, 2012CB944700); National Natural Science Foundation of China (81200419); Shandong Province Excellent Young and Middle-Aged Scientists Research Awards Fund (BS2013YY012); Key Program for Basic Research of the Science and Technology Commission of Shanghai Municipality, China (12JC1405800); China Postdoctoral Science Foundation Funded Project (2012M511522, 2013T60677); and Postdoctoral Innovation Foundation of Shandong Province (201203043). The authors thank all of their colleagues for technical support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All of the authors have no conflict of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhao, S., Xu, H., Cui, Y. et al. Metabolic actions of insulin in ovarian granulosa cells were unaffected by hyperandrogenism. Endocrine 53, 823–830 (2016). https://doi.org/10.1007/s12020-016-0949-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-016-0949-y