Abstract
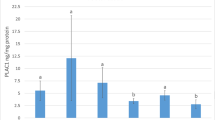
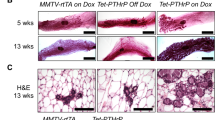
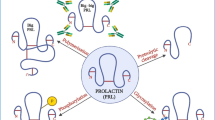
Lactation is an important event in all-mammalian species. To investigate the role of pregnancy-associated plasma protein (PAPP)-A in lactogenesis, we determined (i) PAPP-A expression in mouse mammary glands and (ii) the biological functions of PAPP-A in mammary epithelial cells. PAPP-A mRNA level was low during early mid pregnancy and increased during mid-late pregnancy, and then slightly decreased during lactation. Cell proliferation signals, but not differentiation, increased PAPP-A mRNA expression in HC11 mammary epithelial cells. Treatment of recombinant PAPP-A protein stimulated HC11 cell proliferation and suppressed the expression of β-casein mRNA, which is one of the milk proteins and cell differentiation marker. Surprisingly, in forcing expression experiment, PAPP-A increased β-casein mRNA expression. Our data suggest that PAPP-A has different roles on intracellular expressing and extracellular treatment to mammary epithelial cells. Taken together, in early pregnancy, circulating PAPP-A protein might be supplied from other organs and stimulates mammary gland growth. In contrast, during mid-late pregnancy, local PAPP-A expression begins and enhances cell differentiation within mammary epithelial cell.




Similar content being viewed by others
References
K. Joshi, J.T. Ellis, C.M. Hughes, P. Monaghan, A.M. Neville, Cellular proliferation in the rat mammary gland during pregnancy and lactation. Lab. Invest. 54, 52–61 (1986)
S.R. Wellings, K.B. DeOme, D.R. Pitelka, Electron microscopy of milk secretion in the mammary gland of the C3H/Crg1 mouse; I, Cytomorphology of the prelactating and the lactating gland. J. Natl Cancer Inst. 25, 393–421 (1960)
T.M. Lin, S.P. Halbert, W.N. Spellacy, Measurement of pregnancy-associated plasma proteins during human gestation. J. Clin. Invest. 54, 576–582 (1974)
J. Massé, Y. Giguère, A. Kharfi, J. Girouard, J.C. Forest, Pathophysiology and maternal biologic markers of preeclampsia. Endocrine 19, 113–125 (2002)
G.C. Smith, E.J. Stenhouse, J.A. Crossley, D.A. Aitken, A.D. Cameron et al., Early pregnancy levels of pregnancy-associated plasma protein a and the risk of intrauterine growth restriction, premature birth, preeclampsia, and stillbirth. J. Clin. Endocrinol. Metab. 87, 1762–1767 (2002)
L.K. Proctor, M. Toal, S. Keating, D. Chitayat, N. Okun et al., Placental size and the prediction of severe early-onset intrauterine growth restriction in women with low pregnancy-associated plasma protein-A. Ultrasound Obstet. Gynecol. 34, 274–282 (2009)
A. Bayes-Genis, C.A. Conover, M.T. Overgaard, K.R. Bailey, M. Christiansen et al., Pregnancy-associated plasma protein A as a marker of acute coronary syndromes. N. Engl. J. Med. 345, 1022–1029 (2001)
M.T. Overgaard, E.S. Sorensen, D. Stachowiak, H.B. Boldt, L. Kristensen et al., Complex of pregnancy-associated plasma protein-A and the proform of eosinophil major basic protein. Disulfide structure and carbohydrate attachment. J. Biol. Chem. 278, 2106–2117 (2003)
P. Monget, S. Mazerbourg, T. Delpuech, M.C. Maurel, S. Manière et al., Pregnancy-associated plasma protein-A is involved in insulin-like growth factor binding protein-2 (IGFBP-2) proteolytic degradation in bovine and porcine preovulatory follicles: identification of cleavage site and characterization of IGFBP-2 degradation. Biol. Reprod. 68, 77–86 (2003)
J.B. Lawrence, C. Oxvig, M.T. Overgaard, L. Sottrup-Jensen, G.J. Gleich et al., The insulin-like growth factor (IGF)-dependent IGF binding protein-4 protease secreted by human fibroblasts is pregnancy-associated plasma protein-A. Proc. Natl Acad. Sci. USA 96, 3149–3153 (1999)
L.S. Laursen, M.T. Overgaard, R. Soe, H.B. Boldt, L. Sottrup-Jensen et al., Pregnancy-associated plasma protein-A (PAPP-A) cleaves insulin-like growth factor binding protein (IGFBP)-5 independent of IGF: implications for the mechanism of IGFBP-4 proteolysis by PAPP-A. FEBS Lett. 504, 36–40 (2001)
I. Varela-Nieto, M. Hartl, I. Gorospe, Y. León, Anti-apoptotic actions of insulin-like growth factors: lessons from development and implications in neoplastic cell transformation. Curr. Pharm. Des. 13, 687–703 (2007)
A.M. Arafat, M.O. Weickert, J. Frystyk, J. Spranger, C. Schöfl et al., The role of insulin-like growth factor (IGF) binding protein-2 in the insulin-mediated decrease in IGF-I bioactivity. J. Clin. Endocrinol. Metab. 94, 5093–5101 (2009)
K.A. Woods, F. Dastot, M.A. Preece, A.J. Clark, M.C. Postel-Vinay et al., Phenotype: genotype relationships in growth hormone insensitivity syndrome. J. Clin. Endocrinol. Metab. 82, 3529–3535 (1997)
G. Bonapace, D. Concolino, S. Formicola, P. Strisciuglio, A novel mutation in a patient with insulin-like growth factor 1 (IGF1) deficiency. J. Med. Genet. 40, 913–917 (2003)
M.J. Walenkamp, M. Karperien, A.M. Pereira, Y. Hilhorst-Hofstee, J. van Doorn et al., Homozygous and heterozygous expression of a novel insulin-like growth factor-I mutation. J. Clin. Endocrinol. Metab. 90, 2855–2864 (2005)
M.J. Walenkamp, J.M. Wit, Genetic disorders in the GH IGF-I axis in mouse and man. Eur. J. Endocrinol. 157(Suppl 1), S15–S26 (2007)
I. Netchine, S. Azzi, M. Houang, D. Seurin, L. Perin et al., Partial primary deficiency of insulin-like growth factor (IGF)-I activity associated with IGF1 mutation demonstrates its critical role in growth and brain development. J. Clin. Endocrinol. Metab. 94, 3913–3921 (2009)
M. Ross, G.L. Francis, L. Szabo, J.C. Wallace, F.J. Ballard, Insulin-like growth factor (IGF)-binding proteins inhibit the biological activities of IGF-1 and IGF-2 but not des-(1–3)-IGF-1. Biochem. J. 258, 267–272 (1989)
C.A. Conover, L.K. Bale, M.T. Overgaard, E.W. Johnstone, U.H. Laursen et al., Metalloproteinase pregnancy-associated plasma protein A is a critical growth regulatory factor during fetal development. Development 131, 1187–1194 (2004)
D. Phang, M. Rehage, B. Bonafede, D. Hou, W. Xing et al., Inactivation of insulin-like-growth factors diminished the anabolic effects of pregnancy-associated plasma protein-A (PAPP-A) on bone in mice. Growth Horm. IGF Res. 20, 192–200 (2010)
X. Qin, J.E. Wergedal, M. Rehage, K. Tran, J. Newton et al., Pregnancy-associated plasma protein-A increases osteoblast proliferation in vitro and bone formation in vivo. Endocrinology 147, 5653–5661 (2006)
M.S. Sheppard, R.M. Bala, Growth hormone secretion during pregnancy: altered effects of growth hormone releasing factor and insulin-like growth factor-I in vitro. Horm. Res. 27, 205–210 (1987)
S.G. Bonnette, D.L. Hadsell, Targeted disruption of the IGF-I receptor gene decreases cellular proliferation in mammary terminal end buds. Endocrinology 142, 4937–4945 (2001)
D.J. Flint, E. Tonner, G.J. Allan, Insulin-like growth factor binding proteins: IGF-dependent and -independent effects in the mammary gland. J. Mammary Gland Biol. Neoplasia 5, 65–73 (2000)
H. Chander, M. Halpern, L. Resnick-Silverman, J.J. Manfredi, D. Germain, Skp2B overexpression alters a prohibitin-p53 axis and the transcription of PAPP-A, the protease of insulin-like growth factor binding protein 4. PLoS ONE 6, e22456 (2011)
T. Galbaugh, M.G. Cerrito, C.C. Jose, M.L. Cutler, EGF-induced activation of Akt results in mTOR-dependent p70S6 kinase phosphorylation and inhibition of HC11 cell lactogenic differentiation. BMC Cell Biol. 7, 34 (2006)
P. Accornero, S. Miretti, L.S. Cucuzza, E. Martignani, M. Baratta, Epidermal growth factor and hepatocyte growth factor cooperate to enhance cell proliferation, scatter, and invasion in murine mammary epithelial cells. J. Mol. Endocrinol. 44, 115–125 (2010)
R.K. Ball, A. Ziemiecki, C.A. Schönenberger, E. Reichmann, S.M. Redmond et al., v-myc alters the response of a cloned mouse mammary epithelial cell line to lactogenic hormones. Mol. Endocrinol. 2, 133–142 (1988)
K. Nagaoka, T. Tanaka, K. Imakawa, S. Sakai, Involvement of RNA binding proteins AUF1 in mammary gland differentiation. Exp. Cell Res. 313, 2937–2945 (2007)
T. Tanaka, S. Haneda, K. Imakawa, S. Sakai, K. Nagaoka, A microRNA, miR-101a, controls mammary gland development by regulating cyclooxygenase-2 expression. Differentiation 77, 181–187 (2008)
T.M. Lin, S.P. Halbert, Placental localization of human pregnancy-associated plasma proteins. Science 193, 1249–1252 (1976)
T.M. Lin, S.P. Halbert, D. Kiefer, Quantitative analysis of pregnancy-associated plasma proteins in human placenta. J. Clin. Invest. 57, 466–472 (1976)
T.M. Lin, S.P. Halbert, W.N. Spellacy, Relation of obstetric parameters to the concentrations of four pregnancy-associated plasma proteins at term in normal gestation. Am. J. Obstet. Gynecol. 125, 17–24 (1976)
R. Smith, M.A. Thomson, W. Cooper, The relationship between changing values of pregnancy-associated plasma protein-A in late pregnancy and the onset of labour. Placenta 2, 143–148 (1981)
M.T. Overgaard, J. Haaning, H.B. Boldt, I.M. Olsen, L.S. Laursen, Expression of recombinant human pregnancy-associated plasma protein-A and identification of the proform of eosinophil major basic protein as its physiological inhibitor. J. Biol. Chem. 275, 31128–31133 (2000)
E.S. Henson, S.B. Gibson, Surviving cell death through epidermal growth factor (EGF) signal transduction pathways: implications for cancer therapy. Cell. Signal. 18, 2089–2097 (2006)
C.J. Watson, T.G. Burdon, Prolactin signal transduction mechanisms in the mammary gland: the role of the Jak/Stat pathway. Rev. Reprod. 1, 1–5 (1996)
P.J. Coffer, A. van Puijenbroek, B.M. Burgering, M. Klop-de Jonge, L. Koenderman et al., Insulin activates Stat3 independently of p21ras-ERK and PI-3K signal transduction. Oncogene 15, 2529–2539 (1997)
S.H. Shim, J.H. Hah, S.Y. Hwang, D.S. Heo, M.W. Sung, Dexamethasone treatment inhibits VEGF production via suppression of STAT3 in a head and neck cancer cell line. Oncol. Rep. 23, 1139–1143 (2010)
G.J. Allan, J. Beattie, D.J. Flint, The role of IGFBP-5 in mammary gland development and involution. Domest. Anim. Endocrinol. 27, 257–266 (2004)
Acknowledgments
The authors would like to thank Prof. Nobuyuki Miyasaka and Dr. Yoshinori Nonomura for valuable suggestions and comments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nakasato, M., Kohsaka, H., Mizutani, T. et al. Pregnancy-associated plasma protein (PAPP)-A expressed in the mammary gland controls epithelial cell proliferation and differentiation. Endocrine 43, 387–393 (2013). https://doi.org/10.1007/s12020-012-9766-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-012-9766-0