Abstract
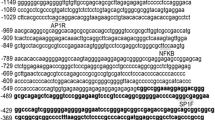
Galectin-3 is expressed in a cell-type specific manner in human pituitary tumors and may have a role in pituitary tumor development. In this study, we hypothesized that Galectin-3 is regulated by RUNX proteins in pituitary tumors. Transcription factor prediction programs revealed several putative binding sites in the LGALS3 (Galectin-3 gene) promoter region. A human pituitary cell line HP75 was used as a model to study LGALS3 and RUNX interactions using Chromatin immunoprecipitation assay and electrophoresis mobility shift assay. Two binding sites for RUNX1 and one binding site for RUNX2 were identified in the LGALS3 promoter region. LGALS3 promoter was further cloned into a luciferase reporter, and the experiments showed that both RUNX1 and RUNX2 upregulated LGALS3. Knock-down of either RUNX1 or RUNX2 by siRNA resulted in a significant downregulation of Galectin-3 expression and decreased cell proliferation in the HP 75 cell line. Immunohistochemistry showed a close correlation between Galectin-3 expression and RUNX1/RUNX2 level in pituitary tumors. These results demonstrate a novel binding target for RUNX1 and RUNX2 proteins and suggest that Galectin-3 is regulated by RUNX1 and RUNX2 in human pituitary tumor cells by direct binding to the promoter region of LGALS3 and thus may contribute to pituitary tumor progression.




Similar content being viewed by others
References
M.P. Shekhar, P. Nangia-Makker, L. Tait, F. Miller, A. Raz, Alterations in galectin-3 expression and distribution correlate with breast cancer progression: functional analysis of galectin-3 in breast epithelial-endothelial interactions. Am. J. Pathol. 165, 1931–1941 (2004)
H.C. Gong, Y. Honjo, P. Nangia-Makker, V. Hogan, N. Mazurak, R.S. Bresalier, A. Raz, The NH2 terminus of galectin-3 governs cellular compartmentalization and functions in cancer cells. Cancer Res. 59, 6239–6245 (1999)
F.T. Liu, G.A. Rabinovich, Galectins as modulators of tumour progression. Nat. Rev. Cancer 5, 29–41 (2005)
K. Yamaoka, K. Mishima, Y. Nagashima, A. Asai, Y. Sanai, T. Kirino, Expression of galectin-1 mRNA correlates with the malignant potential of human gliomas and expression of antisense galectin-1 inhibits the growth of 9 glioma cells. J. Neurosci. Res. 59, 722–730 (2000)
Y. Takenaka, H. Inohara, T. Yoshii, K. Oshima, S. Nakahara, S. Akahani, Y. Honjo, Y. Yamamoto, A. Raz, T. Kubo, Malignant transformation of thyroid follicular cells by galectin-3. Cancer Lett. 195, 111–119 (2003)
R.C. Hughes, The galectin family of mammalian carbohydrate-binding molecules. Biochem. Soc. Trans. 25, 1194–1198 (1997)
R.C. Hughes, Secretion of the galectin family of mammalian carbohydrate-binding proteins. Biochim. Biophys. Acta 1473, 172–185 (1999)
D. Riss, L. Jin, X. Qian, J. Bayliss, B.W. Scheithauer, W.F. Young Jr., S. Vidal, K. Kovacs, A. Raz, R.V. Lloyd, Differential expression of galectin-3 in pituitary tumors. Cancer Res. 63, 2251–2255 (2003)
K.H. Ruebel, L. Jin, X. Qian, B.W. Scheithauer, K. Kovacs, N. Nakamura, H. Zhang, A. Raz, R.V. Lloyd, Effects of DNA methylation on galectin-3 expression in pituitary tumors. Cancer Res. 65, 1136–1140 (2005)
M. Stock, H. Schafer, S. Stricker, G. Gross, S. Mundlos, F. Otto, Expression of galectin-3 in skeletal tissues is controlled by Runx2. J. Biol. Chem. 278, 17360–17367 (2003)
A. Costessi, A. Pines, P. D’Andrea, M. Romanello, G. Damante, L. Cesaratto, F. Quadrifoglio, L. Moro, G. Tell, Extracellular nucleotides activate Runx2 in the osteoblast-like HOBIT cell line: a possible molecular link between mechanical stress and osteoblasts’ response. Bone 36, 418–432 (2005)
L. Jin, E. Kulig, X. Qian, B.W. Scheithauer, N.L. Eberhardt, R.V. Lloyd, A human pituitary adenoma cell line proliferates and maintains some differentiated functions following expression of SV40 large T antigen. Endocr. Pathol. 9, 169–184 (1998)
K. Cartharius, K. Frech, K. Grote, B. Klocke, M. Haltmeier, A. Klingenhoff, M. Frisch, M. Bayerlein, T. Werner, MatInspector and beyond: promoter analysis based on transcription factor binding sites. Bioinformatics 21, 2933–2942 (2005)
T. Tsunoda, T. Takagi, Estimating transcription factor bindability on DNA. Bioinformatics 15, 622–630 (1999)
D. Levanon, V. Negreanu, Y. Bernstein, I. Bar-Am, L. Avivi, Y. Groner, AML1, AML2, and AML3, the human members of the runt domain gene-family: cDNA structure, expression, and chromosomal localization. Genomics 23, 425–432 (1994)
Y. Kamachi, E. Ogawa, M. Asano, S. Ishida, Y. Murakami, M. Satake, Y. Ito, K. Shigesada, Purification of a mouse nuclear factor that binds to both the A and B cores of the polyomavirus enhancer. J. Virol. 64, 4808–4819 (1990)
I.N. Melnikova, B.E. Crute, S. Wang, N.A. Speck, Sequence specificity of the core-binding factor. J. Virol. 67, 2408–2411 (1993)
A.L. Zaiman, A.F. Lewis, B.E. Crute, N.A. Speck, J. Lenz, Transcriptional activity of core binding factor-alpha (AML1) and beta subunits on murine leukemia virus enhancer cores. J. Virol. 69, 2898–2906 (1995)
G. Huang, K. Shigesada, K. Ito, H.J. Wee, T. Yokomizo, Y. Ito, Dimerization with PEBP2beta protects RUNX1/AML1 from ubiquitin-proteasome-mediated degradation. EMBO J. 20, 723–733 (2001)
Y.W. Zhang, N. Yasui, K. Ito, G. Huang, M. Fujii, J. Hanai, H. Nogami, T. Ochi, K. Miyazono, Y. Ito, A RUNX2/PEBP2alpha A/CBFA1 mutation displaying impaired transactivation and Smad interaction in cleidocranial dysplasia. Proc. Natl. Acad. Sci. USA 97, 10549–10554 (2000)
J. Kononen, L. Bubendorf, A. Kallioniemi, M. Barlund, P. Schraml, S. Leighton, J. Torhorst, M.J. Mihatsch, G. Sauter, O.P. Kallioniemi, Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat. Med. 4, 844–847 (1998)
H. Miyoshi, M. Ohira, K. Shimizu, K. Mitani, H. Hirai, T. Imai, K. Yokoyama, E. Soeda, M. Ohki, Alternative splicing and genomic structure of the AML1 gene involved in acute myeloid leukemia. Nucleic Acids Res. 23, 2762–2769 (1995)
M.M. Kadrofske, K.P. Openo, J.L. Wang, The human LGALS3 (galectin-3) gene: determination of the gene structure and functional characterization of the promoter. Arch. Biochem. Biophys. 349, 7–20 (1998)
Y. Yamaguchi, M. Kurokawa, Y. Imai, K. Izutsu, T. Asai, M. Ichikawa, G. Yamamoto, E. Nitta, T. Yamagata, K. Sasaki, K. Mitani, S. Ogawa, S. Chiba, H. Hirai, AML1 is functionally regulated through p300-mediated acetylation on specific lysine residues. J. Biol. Chem. 279, 15630–15638 (2004)
Y.W. Zhang, S.C. Bae, G. Huang, Y.X. Fu, J. Lu, M.Y. Ahn, Y. Kanno, T. Kanno, Y. Ito, A novel transcript encoding an N-terminally truncated AML1/PEBP2 alphaB protein interferes with transactivation and blocks granulocytic differentiation of 32Dcl3 myeloid cells. Mol. Cell. Biol. 17, 4133–4145 (1997)
T.K. Howcroft, J.D. Weissman, A. Gegonne, D.S. Singer, A T lymphocyte-specific transcription complex containing RUNX1 activates MHC class I expression. J. Immunol. 174, 2106–2115 (2005)
T. Fukumori, N. Oka, Y. Takenaka, P. Nangia-Makker, E. Elsamman, T. Kasai, M. Shono, H.O. Kanayama, J. Ellerhorst, R. Lotan, A. Raz, Galectin-3 regulates mitochondrial stability and antiapoptotic function in response to anticancer drug in prostate cancer. Cancer Res. 66, 3114–3119 (2006)
N. Oka, S. Nakahara, Y. Takenaka, T. Fukumori, V. Hogan, H.O. Kanayama, T. Yanagawa, A. Raz, Galectin-3 inhibits tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by activating Akt in human bladder carcinoma cells. Cancer Res. 65, 7546–7553 (2005)
M. Volante, F. Bozzalla-Cassione, F. Orlandi, M. Papotti, Diagnostic role of galectin-3 in follicular thyroid tumors. Virchows Arch. 444, 309–312 (2004)
F. Roncaroli, B.W. Scheithauer, G. Cenacchi, E. Horvath, K. Kovacs, R.V. Lloyd, P. Abell-Aleff, M. Santi, A.J. Yates, ‘Spindle cell oncocytoma’ of the adenohypophysis: a tumor of folliculostellate cells? Am. J. Surg. Pathol. 26, 1048–1055 (2002)
P. Ducy, T. Schinke, G. Karsenty, The osteoblast: a sophisticated fibroblast under central surveillance. Science 289, 1501–1504 (2000)
B.E. Crute, A.F. Lewis, Z. Wu, J.H. Bushweller, N.A. Speck, Biochemical and biophysical properties of the core-binding factor alpha2 (AML1) DNA-binding domain. J. Biol. Chem. 271, 26251–26260 (1996)
T. Kanno, Y. Kanno, L.F. Chen, E. Ogawa, W.Y. Kim, Y. Ito, Intrinsic transcriptional activation-inhibition domains of the polyomavirus enhancer binding protein 2/core binding factor alpha subunit revealed in the presence of the beta subunit. Mol. Cell. Biol. 18, 2444–2454 (1998)
H. Kagoshima, K. Shigesada, M. Satake, Y. Ito, H. Miyoshi, M. Ohki, M. Pepling, P. Gergen, The Runt domain identifies a new family of heteromeric transcriptional regulators. Trends Genet. 9, 338–341 (1993)
Q. Wang, T. Stacy, M. Binder, M. Marin-Padilla, A.H. Sharpe, N.A. Speck, Disruption of the Cbfa2 gene causes necrosis and hemorrhaging in the central nervous system and blocks definitive hematopoiesis. Proc. Natl. Acad. Sci. USA 93, 3444–3449 (1996)
T. Okuda, J. van Deursen, S.W. Hiebert, G. Grosveld, J.R. Downing, AML1, the target of multiple chromosomal translocations in human leukemia, is essential for normal fetal liver hematopoiesis. Cell 84, 321–330 (1996)
T. Komori, H. Yagi, S. Nomura, A. Yamaguchi, K. Sasaki, K. Deguchi, Y. Shimizu, R.T. Bronson, Y.H. Gao, M. Inada, M. Sato, R. Okamoto, Y. Kitamura, S. Yoshiki, T. Kishimoto, Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89, 755–764 (1997)
F. Otto, A.P. Thornell, T. Crompton, A. Denzel, K.C. Gilmour, I.R. Rosewell, G.W. Stamp, R.S. Beddington, S. Mundlos, B.R. Olsen, P.B. Selby, M.J. Owen, Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell 89, 765–771 (1997)
D. Levanon, D. Bettoun, C. Harris-Cerruti, E. Woolf, V. Negreanu, R. Eilam, Y. Bernstein, D. Goldenberg, C. Xiao, M. Fliegauf, E. Kremer, F. Otto, O. Brenner, A. Lev-Tov, Y. Groner, The Runx3 transcription factor regulates development and survival of TrkC dorsal root ganglia neurons. EMBO J. 21, 3454–3463 (2002)
K. Inoue, S. Ozaki, T. Shiga, K. Ito, T. Masuda, N. Okado, T. Iseda, S. Kawaguchi, M. Ogawa, S.C. Bae, N. Yamashita, S. Itohara, N. Kudo, Y. Ito, Runx3 controls the axonal projection of proprioceptive dorsal root ganglion neurons. Nat. Neurosci. 5, 946–954 (2002)
H.M. Robinson, Z.J. Broadfield, K.L. Cheung, L. Harewood, R.L. Harris, G.R. Jalali, M. Martineau, A.V. Moorman, K.E. Taylor, S. Richards, C. Mitchell, C.J. Harrison, Amplification of AML1 in acute lymphoblastic leukemia is associated with a poor outcome. Leukemia 17, 2249–2250 (2003)
G.L. Barnes, K.E. Hebert, M. Kamal, A. Javed, T.A. Einhorn, J.B. Lian, G.S. Stein, L.C. Gerstenfeld, Fidelity of Runx2 activity in breast cancer cells is required for the generation of metastases-associated osteolytic disease. Cancer Res. 64, 4506–4513 (2004)
A. Kilbey, K. Blyth, S. Wotton, A. Terry, A. Jenkins, M. Bell, L. Hanlon, E.R. Cameron, J.C. Neil, Runx2 disruption promotes immortalization and confers resistance to oncogene-induced senescence in primary murine fibroblasts. Cancer Res. 67, 11263–11271 (2007)
V. Vladimirova, A. Waha, K. Luckerath, P. Pesheva, R. Probstmeier, Runx2 is expressed in human glioma cells and mediates the expression of galectin-3. J. Neurosci. Res. 86, 2450–2461 (2008)
Acknowledgments
This research was supported in part by NIH Grant CA90249 and a Grant from the Jarislowsky Foundation to RVL) and NIH Grant R37CA46120-19 (to AR).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhang, HY., Jin, L., Stilling, G.A. et al. RUNX1 and RUNX2 upregulate Galectin-3 expression in human pituitary tumors. Endocr 35, 101–111 (2009). https://doi.org/10.1007/s12020-008-9129-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12020-008-9129-z