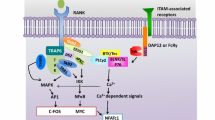
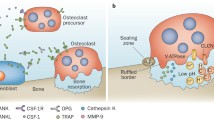
Abstract
Osteoclasts are multinucleated cells derived from mononuclear phagocyte precursors (monocytes, macrophages); in the canonical pathway of osteoclastogenesis, these cells fuse and differentiate to form specialised bone-resorbing osteoclasts in the presence of receptor activator for nuclear factor kappa B ligand (RANKL). Non-canonical pathways of osteoclastogenesis have been described in which several cytokines and growth factors are able to substitute for RANKL. These humoral factors can generally be divided into those which, like RANKL, are tumour necrosis family (TNF) superfamily members and those which are not; the former include TNFα lymphotoxin exhibiting inducible expression and competing with herpes simplex virus glycoprotein D for herpesvirus entry mediator, a receptor expressed by T lymphocytes (LIGHT), a proliferation inducing ligand (APRIL) and B cell activating factor (BAFF); the latter include transforming growth factor beta (TGF-β), interleukin-6 (IL-6), IL-8, IL-11, nerve growth factor (NGF), insulin-like growth factor-I (IGF-I) and IGF-II. This review summarises the evidence for these RANKL substitutes in inducing osteoclast differentiation from tissue-derived and circulating mononuclear phagocytes. It also assesses the role these factors are likely to play in promoting the pathological bone resorption seen in many inflammatory and neoplastic lesions of bone and joint including rheumatoid arthritis, aseptic implant loosening and primary and secondary tumours of bone.

Similar content being viewed by others
References
Knowles HJ, Athanasou NA (2009) Canonical & non-canonical pathways of osteoclast formation. Histol Histopathol 24:337–346
Athanasou NA (2011) The osteoclast—what’s new? Skelet Radiol 40:1137–1140
Massey HM, Flanagan AM (1999) Human osteoclasts derive from CD14-positive monocytes. Br J Haematol 106:167–170
Fujikawa Y, Quinn JM, Sabokbar A, McGee JO’D, Athanasou NA (1996) The human osteoclast precursor circulates in the monocyte fraction. Endocrinology 137:4058–4060
Walker DG (1993) Bone resorption restored in osteopetrotic mice by transplants of normal bone marrow and spleen cells. Clin Orthop Relat Res 294:4–6
Faust J, Lacey DL, Hunt P et al (1999) Osteoclast markers accumulate on cells developing from human peripheral blood mononuclear precursors. J Cell Biochem 72:67–80
Lacey DL, Timms E, Tan HL et al (1998) Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 93:165–176
Yasuda H, Shima N, Nakagawa N et al (1998) Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A 95:3597–3602
Lum L, Wong BR, Josien R et al (1999) Evidence for a role of a tumour necrosis factor-alpha (TNF-alpha)-converting enzyme-like protease in shedding of TRANCE, a TNF family member involved in osteoclastogenesis and dendritic cell survival. J Biol Chem 274:13613–13618
Kong YY, Yoshida H, Sarosi I et al (1999) OPGL is a key regulator of osteoclastogenesis, lymphocyte development and lymph-node organogenesis. Nature 397:315–323
Kitazawa R, Kitazawa S (2002) Vitamin D(3) augments osteoclastogenesis via vitamin D-responsive element of mouse RANKL gene promoter. Biochem Biophys Res Commun 290:650–655
Horwood NJ, Elliott J, Martin TJ, Gillespie MT (1998) Osteotropic agents regulate the expression of osteoclast differentiation factor and osteoprotegerin in osteoblastic stromal cells. Endocrinology 139:4743–4746
Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S (1999) Interleukin-1beta and tumour necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone 25:255–259
Atkins GJ, Bouralexis S, Haynes DR et al (2001) Osteoprotegerin inhibits osteoclast formation and bone resorbing activity in giant cell tumors of bone. Bone 28:370–377
Fuller K, Wong B, Fox S, Choi Y, Chambers TJ (1998) TRANCE is necessary and sufficient for osteoblast-mediated activation of bone resorption in osteoclasts. J Exp Med 188:997–1001
Li J, Sarosi I, Yan XQ et al (2000) RANK is the intrinsic hematopoietic cell surface receptor that controls osteoclastogenesis and regulation of bone mass and calcium metabolism. Proc Natl Acad Sci U S A 97:1566–1571
Khosla S (2001) Minireview: the OPG/RANKL/RANK system. Endocrinology 142:5050–5055
Kim N, Odgren PR, Kim DK, Marks SC Jr, Choi Y (2000) Diverse roles of the tumour necrosis factor family member TRANCE in skeletal physiology revealed by TRANCE deficiency and partial rescue by a lymphocyte-expressed TRANCE transgene. Proc Natl Acad Sci U S A 97:10905–10910
Odgren PR, Kim N, MacKay CA, Mason-Savas A, Choi Y, Marks SC Jr (2003) The role of RANKL (TRANCE/TNFSF11), a tumour necrosis factor family member, in skeletal development: effects of gene knockout and transgenic rescue. Connect Tissue Res 44(Suppl 1):264–271
Dougall WC, Glaccum M, Charrier K et al (1999) RANK is essential for osteoclast and lymph node development. Genes Dev 13:2412–2424
Udagawa N, Takahashi N, Yasuda H et al (2000) Osteoprotegerin produced by osteoblasts is an important regulator in osteoclast development and function. Endocrinology 141:3478–3484
Hofbauer LC (1999) Osteoprotegerin ligand and osteoprotegerin: novel implications for osteoclast biology and bone metabolism. Eur J Endocrinol 141:195–210
Mizuno A, Amizuka N, Irie K et al (1998) Severe osteoporosis in mice lacking osteoclastogenesis inhibitory factor/osteoprotegerin. Biochem Biophys Res Commun 247:610–615
Bucay N, Sarosi I, Dunstan CR et al (1998) Osteoprotegerin-deficient mice develop early onset osteoporosis and arterial calcification. Genes Dev 12:1260–1268
Wada T, Nakashima T, Hiroshi N, Penninger JM (2006) RANKL-RANK signaling in osteoclastogenesis and bone disease. Trends Mol Med 2006(12):17–25
Lomaga MA, Yeh WC, Sarosi I et al (1999) TRAF6 deficiency results in osteopetrosis and defective interleukin-1, CD40, and LPS signalling. Genes Dev 13:1015–1024
Kanazawa K, Kudo A (2000) TRAF2 is essential for TNF-alpha-induced osteoclastogenesis. J Bone Miner Res 20:840–847
Kanazawa K, Azuma Y, Nakano H, Kudo A (2003) TRAF5 functions in both RANKL- and TNF alpha-induced osteoclastogenesis. J Bone Miner Res 18:443–450
Nakamura I, Takahashi N, Sasaki T et al (1995) A specific inhibitor of phosphatidylinositol-3 kinase, blocks osteoclastic bone resorption. FEBS Lett 361:79–84
Iotsova V, Caamaño J, Loy J, Yang Y, Lewin A, Bravo R (1997) Osteopetrosis in mice lacking NF-kappaB1 and NF-kappaB2. Nat Med 3:1285–1289
Soriano P, Montgomery C, Geske R, Bradley A (1991) Targeted disruption of the c-src proto-oncogene leads to osteopetrosis in mice. Cell 64:693–702
Takeshita S, Namba N, Zhao J et al (2002) SHIP-deficient mice are severely osteoporotic due to increased numbers of hyper-resorptive osteoclasts. Nat Med 8:943–949
David JP, Sabapathy K, Hoffmann O, Idarraga MH, Wagner EF (2002) JNK1 modulates osteoclastogenesis through both c-Jun phosphorylation-dependent and -independent mechanisms. J Cell Sci 115:4317–4325
Kenner L, Hoebertz A, Beil FT et al (2004) Mice lacking JunB are osteopenic due to cell-autonomous osteoblast and osteoclast defects. J Cell Biol 164:613–623
Wagner EF (2002) Functions of AP1 (Fos/Jun) in bone development. Ann Rheum Dis 61(Suppl 2):40–42
Fleischmann A, Hafezi F, Elliott C, Remé CE, Rüther U, Wagner EF (2000) Fra-1 replaces c-Fos-dependent functions in mice. Genes Dev 14:2695–2700
Felix R, Hofstetter W, Wetterwald A, Cecchini MG, Fleisch H (1994) Role of colony-stimulating factor-1 in bone metabolism. J Cell Biochem 55:340–349
Flanagan AM, Lader CS (1998) Update on the biologic effects of macrophage colony-stimulating factor. Curr Opin Hematol 5:181–185
Motoyoshi K (1998) Biological activities and clinical application of M-CSF. Int J Hematol 67:109–122
Sherr CJ, Rettenmier CW, Sacca R, Roussel MF, Look AT, Stanley ER (1985) The c-fms proto-oncogene product is related to the receptor for the mononuclear phagocyte growth factor, CSF-1. Cell 41:665–676
Felix R, Cecchini MG, Fleisch H (1990) Macrophage colony stimulating factor restores in vivo bone resorption in the op/op osteopetrotic mouse. Endocrinology 127:2592–2594
Yoshida H, Hayashi S, Kunisada T et al (1990) The murine mutation osteopetrosis is in the coding region of the macrophage colony stimulating factor gene. Nature 345:442–444
Mabilleau G, Pascaretti-Grizon F, Basle MF, Chappard D (2012) Depth and volume of resorption induced by osteoclasts generated in the presence of RANKL, TNF-alpha/IL-1 or LIGHT. Cytokine 57(2):294–299
Lam J, Takeshita S, Barker JE, Kanagawa O, Ross FP, Teitelbaum SL (2000) TNF-alpha induces osteoclastogenesis by direct stimulation of macrophages exposed to permissive levels of RANK ligand. J Clin Invest 106:1481–1488
Azuma Y, Kaji K, Katogi R, Takeshita S, Kudo A (2000) Tumour necrosis factor-alpha induces differentiation of and bone resorption by osteoclasts. J Biol Chem 275:4858–4864
Bossen C, Ingold K, Tardivel A et al (2006) Interactions of tumour necrosis factor (TNF) and TNF receptor family members in the mouse and human. J Biol Chem 281:13964–13971
Edwards JR, Sun SG, Locklin R et al (2006) LIGHT (TNFSF14), a novel mediator of bone resorption, is elevated in rheumatoid arthritis. Arthritis Rheum 54:1451–1462
Pasero C, Barbarat B, Just-Landi S et al (2009) A role for HVEM, but not lymphotoxin-beta receptor, in LIGHT-induced tumour cell death and chemokine production. Eur J Immunol 39:2502–2514
Atkins GJ, Haynes DR, Graves SE et al (2000) Expression of osteoclast differentiation signals by stromal elements of giant cell tumors. J Bone Miner Res 15:640–649
Itonaga I, Sabokbar A, Murray DW, Athanasou NA (2000) Effect of osteoprotegerin and osteoprotegerin ligand on osteoclast formation by arthroplasty membrane derived macrophages. Ann Rheum Dis 59:26–31
Tervahartiala T, Koski H, Xu JW, Häyrinen-Immonen R, Hietanen J, Sorsa T, Konttinen YT (2001) Tumor necrosis factor-alpha and its receptors, p55 and p75, in gingiva of adult periodontitis. J Dent Res 80:1535–1539
Brennan F, McInnes IB (2008) Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 118:3537–3545
Adamopoulos IE, Sabokbar A, Wordsworth BP, Carr A, Ferguson DJ, Athanasou NA (2006) Synovial fluid macrophages are capable for osteoclast formation and resorption. J Pathol 208:35–43
Chu CQ, Field M, Feldmann M, Maini RN (1991) Localisation of tumour necrosis factor alpha in synovial tissues and at the cartilage-pannus junction in patients with rheumatoid arthritis. Arthritis Rheum 34:1125–1132
Shaikh RB, Santee S, Granger SW et al (2001) Constitutive expression of LIGHT on T cells leads to lymphocyte activation, inflammation, and tissue destruction. J Immunol 167:6330–6337
Hohmann EL, Elde RP, Rysavy JA, Einzig S, Gebhard RL (1986) Innervation of periosteum and bone by sympathetic vasoactive intestinal peptide-containing nerve fibers. Science 232:868–871
Wang L, Zhou S, Liu B et al (2006) Locally applied nerve growth factor enhances bone consolidation in a rabbit model of mandibular distraction osteogenesis. J Orthop Res 24:2238–2245
Togari A (2002) Adrenergic regulation of bone metabolism: possible involvement of sympathetic innervation of osteoblastic and osteoclastic cells. Microsc Res Tech 58:77–84
Mohan S, Jennings JC, Linkhart TA, Baylink DJ (1988) Primary structure of human skeletal growth factor: homology with human insulin-like growth factor-II. Biochim Biophys Acta 966:44–55
Hemingway F, Taylor R, Knowles HJ, Athanasou NA (2011) RANKL-independent human osteoclast formation with APRIL, BAFF, NGF, IGF I and IGF II. Bone 48:938–944
Yao Z, Xing L, Boyce BF (2009) NF-kappaB p100 limits TNF-induced bone resorption in mice by a TRAF3-dependent mechanism. J Clin Invest 119:3024–3034
Centrella M, Horowitz MC, Wozney JM, McCarthy TL (1994) Transforming growth factor-gene family members and bone. Endocr Rev 15:27–39
Pfeilschifter J, Seyedin SM, Mundy GR (1998) Transforming growth factor beta inhibits bone resorption in fetal rat long bone culture. J Clin Invest 82:680–685
Inoue M, Ross FP, Erdmann JM, Abu-Amer Y, Wei S, Teitelbaum SL (2006) Tumour necrosis factor alpha regulates alpha(v)beta5 integrin expression by osteoclast precursors in vitro and in vivo. Endocrinology 141:284–290
Kudo O, Fujikawa Y, Itonaga I, Sabokbar A, Torisu T, Athanasou NA (2002) Proinflammatory cytokine (TNFalpha/IL-1alpha) induction of human osteoclast formation. J Pathol 198:220–227
Ma T, Miyanishi K, Suen A et al (2004) Human interleukin-1-induced murine osteoclastogenesis is dependent on RANKL, but independent of TNF-α. Cytokine 26:138–144
Wei S, Kitaura H, Zhou P, Ross FP, Teitelbaum SL (2005) IL-1 mediates TNF-induced osteoclastogenesis. J Clin Invest 115:282–290
Teitelbaum SL (2007) Osteoclasts: what do they do and how do they do it? Am J Pathol 170(2):427–435
Mabilleau G, Chappard D, Sabokbar A (2011) Role of the A20-TRAF6 axis in lipopolysaccharide-mediated osteoclastogenesis. J Biol Chem 286(5):3242–3249
Mahoney DJ, Swales C, Athanasou NA et al (2011) TSG-6 inhibits osteoclast activity via an autocrine mechanism and is functionally synergistic with osteoprotegerin. Arthritis Rheum 63(4):1034–1043
Fuller K, Murphy C, Kirstein B, Fox SW, Chambers TJ (2002) TNFα potently activates osteoclasts, through a direct action independent of and strongly synergistic with RANKL. Endocrinology 143:1108–1118
Fuller K, Kirstein B, Chambers TJ (2006) Murine osteoclast formation and function: differential regulation by humoral agents. Endocrinology 147:1979–1985
Iqbal J (2006) Does TNF have anti osteoclastogenic actions? Ann N Y Acad Sci 1068:234–239
Mauri DN, Ebner R, Montgomery RI et al (1998) LIGHT, a new member of the TNF superfamily, and lymphotoxin are ligands for herpesvirus entry mediator. Immunity 8:21–30
Tamada K, Shimozaki K, Chapoval AI et al (2000) LIGHT, a TNF-like molecule, costimulates T cell proliferation and is required for dendritic cell-mediated allogeneic T cell response. J Immunol 164:4105–4110
Montgomery RI, Warner MS, Lum BJ, Spear PG (1996) Herpes simplex virus-1 entry into cells mediated by a novel member of TNF/NGF receptor family. Cell 87:427–436
Zhai Y, Guo R, Hsu TL et al (1998) LIGHT, a novel ligand for lymphotoxin receptor and TR2/HVEM, induces apoptosis and suppresses in vivo tumor formation via gene transfer. J Clin Invest 102:1142–1151
Heo SK, Yun HJ, Park WH, Park SD (2008) NADPH oxidase activation is required for migration by LIGHT in human monocytes. Biochem Biophys Res Commun 371:834–840
Schneider K, Potter KG, Ware CF (2004) Lymphotoxin and LIGHT signaling pathways and target genes. Immunol Rev 202:49–66
Chang TH, Hsieh SL, Chao Y, Chou YC, Lin WW (2005) Proinflammatory effects of LIGHT through HVEM and LTbetaR interactions in cultured human umbilical vein endothelial cells. J Biomed Sci 12:363–375
Kang YM, Kim SY, Kang JH et al (2007) LIGHT up-regulated on B lymphocytes and monocytes in rheumatoid arthritis mediates cellular adhesion and metalloproteinase production by synoviocytes. Arthritis Rheum 56:1106–1117
Ye Q, Fraser CC, Gao W et al (2002) Modulation of LIGHT-HVEM costimulation prolongs cardiac allograft survival. J Exp Med 195:795–800
Rooney IA, Butrovich KD, Glass AA et al (2000) The lymphotoxin-beta receptor is necessary and sufficient for LIGHT-mediated apoptosis of tumor cells. J Biol Chem 275:14307–14315
Granger SW, Rickert S (2003) LIGHT-HVEM signaling and the regulation of T cell-mediated immunity. Cytokine Growth Factor Rev 14:289–296
Marsters SA, Ayres TM, Skubatch M, Gray CL, Rothe M, Ashkenazi A (1997) Herpesvirus entry mediator, a member of the tumor necrosis factor receptor (TNFR) family, interacts with members of the TNFR-associated factor family and activates the transcription factors NF-kappaB and AP-1. J Biol Chem 272:14029–14032
Yang CR, Wang JH, Hsieh SL, Wang SM, Hsu TL, Lin WW (2004) Decoy receptor 3 (DcR3) induces osteoclast formation from monocyte/macrophage lineage precursor cells. Cell Death Differ. Suppl 1:S97-107
Hemingway F, Kashima TG, Knowles HJ, Athanasou NA (2013) Investigation of osteoclastogenic signalling of the RANKL substitute LIGHT. Exp Mol Pathol 94:380–385
Arch RH, Gedrich RW, Thompson CB (1998) Tumour necrosis factor receptor-associated factors (TRAFs) - a family of adapter proteins that regulates life and death. Genes Dev 12:2821–2830
Hahne M, Kataoka T, Schroter M et al (1998) APRIL, a new ligand of the tumor necrosis factor family, stimulates tumor cell growth. J Exp Med 188:1185–1190
Kelly K, Manos E, Jensen G, Nadauld L, Jones DA (2000) APRIL/TRDL-1, a tumor necrosis factor-like ligand, stimulates cell death. Cancer Res 60:1021–1027
Schneider P, MacKay F, Steiner V et al (1999) BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med 189:1747–1756
Bonewald LF (1995) Regulation and regulatory activities of transforming growth factor beta. Crit Rev Eukaryot Gene Exp 9:33–44
Massey HM, Scopes J, Horton MA, Flanagan AM (2002) Transforming growth factor-beta stimulates the osteoclast-forming potential of peripheral blood haematopoietic precursors in a lymphocyte-rich microenvironment. Bone 28:577–582
Beaudreuil J, Mbalaviele G, Cohen-Solal M, Morieux C, de Vernejoul MC, Orcel P (1995) Short-term local injections of transforming growth factor-beta 1 decrease ovariectomy-stimulated osteoclastic resorption in vivo in rat. J Bone Miner Res 10:971–977
Ota K, Quint P, Weivoda MM et al (2013) Transforming growth factor beta 1 induces CXCL16 and leukemia inhibitory factor expression in osteoclasts to modulate migration of osteoblast progenitors. Bone 57:68–75
Itonaga I, Sabokbar A, Sun SG et al (2004) Transforming growth factor-beta induces osteoclast formation in the absence of RANKL. Bone 34:57–64
Takai H, Kanematsu M, Yano K et al (1998) Transforming growth factor-beta stimulates the production of osteoprotegerin/osteoclastogenesis inhibitory factor by bone marrow stromal cells. J Biol Chem 273:27091–27096
Ishimi Y, Miyaura C, Jin CH et al (1990) IL-6 is produced by osteoblasts and induces bone resorption. J Immunol 145:3297–3303
Tamura T, Udagawa N, Takahashi N et al (1993) Soluble interleukin-6 receptor triggers osteoclast formation by interleukin 6. Proc Natl Acad Sci U S A 90:11924–11928
Kudo O, Sabokbar A, Pocock A, Itonaga I, Fujikawa Y, Athanasou NA (2003) Interleukin-6 and interleukin-11 support human osteoclast formation by a RANKL-independent mechanism. Bone 32:1–7
Roodman GD, Kurihara N, Ohsaki Y et al (1992) Interleukin 6. A potential autocrine/paracrine factor in Paget’s disease of bone. J Clin Invest 89:46–52
Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ (2003) Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 33:28–37
Bendre MS, Margulies AG, Walser B et al (2005) Tumour-derived interleukin-8 stimulates osteolysis independent of the receptor activator of nuclear factor-kappaB ligand pathway. Cancer Res 65:11001–11009
Asaumi K, Nakanishi T, Asahara H, Inoue H, Takigawa M (2000) Expression of neurotrophins and their receptors (TRK) during fracture healing. Bone 26:625–633
Frenkel SR, Guerra LA, Mitchell OG, Singh IJ (1990) Nerve growth factor in skeletal tissues of the embryonic chick. Cell Tissue Res 260:507–511
Grills B, Schuijers J (1998) Immunohistochemical localization of nerve growth factor in fractured and unfractured rat bone. Acta Orthop Scand 69:415–419
Hukkanen M, Konttinen Y, Santavirta S et al (1993) Rapid proliferation of calcitonin gene-related peptide-immunoreactive nerves during healing of rat tibial fracture suggests neural involvement in bone growth and remodelling. Neuroscience 54:969–979
Serre CM, Farlay D, Delmas PD, Chenu C (1990) Evidence for a dense and intimate innervation of the bone tissue, including glutamate-containing fibers. Bone 25:623–629
Bautista CM, Mohan S, Baylink DJ (1990) Insulin-like growth factors I and II are present in the skeletal tissues of ten vertebrates. Metabolism 39:96–100
Hayden JM, Mohan S, Baylink DJ (1995) The insulin-like growth factor system and the coupling of formation to resorption. Bone 17:93S–98S
Hill PA, Reynolds JJ, Meikle MC (1995) Osteoblasts mediate insulin-like growth factor-I and -II stimulation of osteoclast formation and function. Endocrinology 136:124–131
Fukuoka H, Aoyama M, Miyazawa K, Asai K, Goto S (2005) Hypoxic stress enhances osteoclast differentiation via increasing IGF2 production by non-osteoclastic cells. Biochem Biophys Res Commun 328:885–894
Mochizuki H, Hakeda Y, Wakatsuki N et al (1992) Insulin-like growth factor-I supports formation and activation of osteoclasts. Endocrinology 131:1075–1080
Nakao K, Aoyama M, Fukuoka H, Fujita M, Miyazawa K, Asai K, Goto S (2009) IGF II modulates the microenvironment for osteoclastogenesis. Biochem Biophys Res Commun 378:462–466
Zhang M, Xuan S, Bouxsein ML et al (2002) Osteoblast-specific knockout of the insulin-like growth factor (IGF) receptor gene reveals an essential role of IGF signalling in bone matrix mineralization. J Biol Chem 277:44005–44012
Wang Y, Nishida S, Elalieh HZ, Long RK, Halloran BP, Bikle DD (2006) Role of IGF-I signaling in regulating osteoclastogenesis. J Bone Miner Res 21:1350–1358
Guicheux J, Heymann D, Rousselle AV et al (1998) Growth hormone stimulatory effects on osteoclastic resorption are partly mediated by insulin-like growth factor I: an in vitro study. Bone 22:25–31
Bautista CM, Baylink DJ, Mohan S (1991) Isolation of a novel insulin-like growth factor (IGF) binding protein from human bone: a potential candidate for fixing IGF-II in human bone. Biochem Biophys Res Commun 176:756–763
Middleton J, Arnott N, Walsh S, Beresford J (1995) Osteoblasts and osteoclasts in adult human osteophyte tissue express the mRNAs for insulin-like growth factors I and II and the type 1 IGF receptor. Bone 16:287–293
Zheng MH, Fan Y, Smith A, Wysocki S, Papadimitriou JM, Wood DJ (1998) Gene expression of monocyte chemoattractant protein-1 in giant cell tumors of bone osteoclastoma: possible involvement in CD68+ macrophage-like cell migration. J Cell Biochem 70:121–129
Franchi A, Benvenuti S, Masi L et al (2001) TGF-beta isoform and receptor expression in giant cell tumor and giant cell lesions of bone. Appl Immunohistochem Mol Morphol 9:170–175
Middleton J, Arnott N, Walsh S, Beresford J (1996) The expression of mRNA for insulin-like growth factors and their receptor in giant cell tumors of human bone. Clin Orthop 322:224–231
Mhawech-Fauceglia P, Kaya G, Sauter G et al (2006) The source of APRIL up-regulation in human solid tumor lesions. J Leukoc Biol 80:697–704
Lau YS, Adamopoulos IE, Sabokbar A, Giele H, Gibbons CL, Athanasou NA (2007) Cellular and humoral mechanisms of osteoclast formation in Ewing’s sarcoma. Br J Cancer 96:1716–1722
Lau YS, Danks L, Sun SG et al (2007) RANKL-dependent and RANKL-independent mechanisms of macrophage-osteoclast differentiation in breast cancer. Breast Cancer Res Treat 105:7–16
Lau YS, Sabokbar A, Giele H, Cerundolo V, Hofstetter W, Athanasou NA (2006) Malignant melanoma and bone resorption. Br J Cancer 94(10):1496–1503
Oranger A, Carbone C, Izzo M, Grano M. (2013) Cellular mechanisms of multiple myeloma bone disease. Clin Dev Immunol. 289458
Brunetti G, Rizzi R, Oranger A et al (2014) LIGHT/TNFSF14 increases osteoclastogenesis and decreases osteoblastogenesis in multiple myeloma-bone disease. Oncotarget 5:12950–12967
Galson DL, Roodman GD (2014) Pathobiology of Paget’s Disease of Bone. J Bone Metab 21:85–98
Hirayama T, Sabokbar A, Itonaga I, Watt-Smith S, Athanasou NA (2014) Cellular and humoral mechanisms of osteoclast formation and bone resorption in Gorham-Stout disease. J Pathol 195:624–630
Neri P, Kumar S, Fulciniti MT et al (2007) Neutralizing B-cell activating factor antibody improves survival and inhibits osteoclastogenesis in a severe combined immunodeficient human multiple myeloma model. Clin Cancer Res 13:5903–5909
Moreaux J, Cremer FW, Reme T et al (2005) The level of TACI gene expression in myeloma cells is associated with a signature of microenvironment dependence versus a plasmablastic signature. Blood 106:1021–1030
Abe M, Kido S, Hiasa M et al (2006) BAFF and APRIL as osteoclast-derived survival factors for myeloma cells: a rationale for TACI-Fc treatment in patients with multiple myeloma. Leukemia 20:1313–1315
Sabokbar A, Kudo O, Athanasou NA (2003) Two distinct cellular mechanisms of osteoclast formation and bone resorption in periprosthetic osteolysis. J Orthop Res 21:73–80
Yang YM, Kim SY, Kang JH et al (2007) LIGHT up regulated on B lymphocytes and monocytes in rheumatoid arthritis mediates cellular adhesion and metalloproteinase production by synoviocytes. Arthritis Rheum 56:1106–1107
Fava RA, Notidis E, Hunt J et al (2003) A role for the lymphotoxin/LIGHT axis in the pathogenesis of murine collagen-induced arthritis. J Immunol 171:115–116
Ishida S, Yamane S, Nakano S et al (2009) The interaction of monocytes with rheumatoid synovial cells is a key step in LIGHT-mediated inflammatory bone destruction. Immunology 128:315–324
Ishida S, Yamane S, Ochi T et al (2003) LIGHT induces cell proliferation and inflammatory responses of rheumatoid arthritis synovial fibroblasts via lymphotoxin beta receptor. J Rheumatol 35:960–968
Pierer M, Brentano F, Rethage J. et al. (2009) The TNF superfamily member LIGHT contributes to survival and activation of synovial fibroblasts in rheumatoid arthritis
Cheung TC, Coppieters K, Sanjo H et al (2010) Polymorphic variants of LIGHT (TNF superfamily −14) alter receptor avidity and bioavailability. J Immunol 185:1949–1958
Mariette X, Roux S, Zhang J et al (2003) The level of BLyS (BAFF) correlates with the titre of autoantibodies in human Sjogren’s syndrome. Ann Rheum Dis 62:168–171
Pers JO, Daridon C, Devauchelle V et al (2005) BAFF overexpression is associated with autoantibody production in autoimmune diseases. Ann N Y Acad Sci 1050:34–39
Dickerson TJ, Suzuki E, Stanecki C, Shin HS, Qui H, Adamopoulos IE (2012) Rheumatoid and pyrophosphate arthritis synovial fibroblasts induce osteoclastogenesis independently of RANKL, TNF and IL-6. J Autoimmun 39:369–376
Adamopoulos IE, Tessmer M, Chao CC et al (2011) IL-23 is critical for induction of arthritis, osteoclast formation, and maintenance of bone mass. J Immunol 187:951–959
Adamopoulos IE, Mellins ED (2015) Alternative pathways of osteoclastogenesis in inflammatory arthritis. Nat Rev Rheumatol 11:189–194
Shin HS, Sarin R, Dixit N, Wu J, Gershwin E, Bowman EP, Adamopoulos IE (2015) Crosstalk among IL-23 and DNAX activating protein of 12 kDa-dependent pathways promotes osteoclastogenesis. J Immunol 194:316–324
Acknowledgments
We would like to thank Sarah Turton for typing the manuscript. NAA and AS are supported by the Oxford NIHR BRU, The Rosetrees Trust, Sarcoma (UK) and the Bone Cancer Research Trust.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Afsie Sabokbar, David Mahoney, Francesca Hemingway and Nicholas Athanasou declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Sabokbar, A., Mahoney, D.J., Hemingway, F. et al. Non-Canonical (RANKL-Independent) Pathways of Osteoclast Differentiation and Their Role in Musculoskeletal Diseases. Clinic Rev Allerg Immunol 51, 16–26 (2016). https://doi.org/10.1007/s12016-015-8523-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-015-8523-6