Abstract
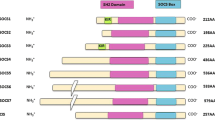
Serine protease inhibitors (serpins) are evolutionary old, structurally conserved molecules which encompass nearly all branches of life. More than 1,000 serpins were characterized to date which are subdivided into 16 subgroups (A–P) according to their common ancestry; among them, 37 are found in humans. Serpins were termed after their capability to inhibit serine proteases, but mounting evidence suggests that they may achieve a greater deal of functions, ranging from embryological growth to synaptic plasticity, development of both myeloid and lymphoid immune cells, and modulation of apoptosis. Serpins are mainly extracellular molecules, although some of them (namely, ov-serpins or clade B serpins) mostly act inside the cells, being either ubiquitously or tissue-specifically expressed. Among newly characterized serpin functions, regulation of cellular proliferation through apoptosis modulation and proteasome disturbance seems to play a major role. Accordingly, several serpins were found to be hyperexpressed in tumor cells. Indeed, apoptosis dysregulation is likely to be a cornerstone in both tumorigenesis and autoimmunity, since uncontrolled cellular viability results in tumor proliferation, while inefficient disposal of apoptotic debris may favor the rescue of autoreactive immune cells. Such a process was widely documented in systemic lupus erythematosus (SLE). Interestingly, alterations in the expression of some serpins, e.g., the ov-serpin SERPINB3, are being unraveled in patients affected with SLE and other autoimmune disorders, suggesting that a failure in serpin function might affect immune homeostasis and self-tolerance, thereby contributing to autoimmunity. Here, we provide an overview of serpin origin, function, and dysfunction, focusing on human serpins and ov-serpins, with a hub on SERPINB3.



Similar content being viewed by others
References
Gettings PG (2002) Serpin structure, mechanism, and function. Chem Rev 102:4751–4804
Huntington JA (2011) Serpin structure, function and dysfunction. J Thromb Haemost 9(Suppl 1):26–34
Rawlings ND, Tolle DP, Barrett AJ (2004) Evolutionary families of peptidase inhibitors. Biochem J 378:705–716
Silverman GA, Bird PI, Carrell RW, Church FC, Coughlin PB, Gettings PG et al (2001) The serpins are an expanding superfamily of structurally similar but functionally diverse proteins. Evolution, mechanism of inhibition, novel functions, and a revised nomenclature. J Biol Chem 276:33293–33296
Law RH, Zhang Q, McGowan S, Buckle AM, Silverman GA, Wong W et al (2006) An overview of the serpin superfamily. Genome Biol 7:216
Mangan MS, Kaiserman D, Bird PI (2008) The role of serpins in vertebrate immunity. Tissue Antigens 72:1–10
Irving JA, Pike RN, Lesk AM, Whisstock JC (2000) Phylogeny of the serpin superfamily: implications of patterns of amino acid conservation for structure and function. Genome Res 10:1845–1864
Silverman GA, Whisstock JC, Askew DJ, Pak SC, Luke CJ, Cataltepe S et al (2004) Human clade B serpins (ov-serpins) belong to a cohort of evolutionarily dispersed intracellular proteinase inhibitor clades that protect cells from promiscuous proteolysis. Cell Mol Life Sci 61:301–325
Bots M, Medema JP (2008) Serpins in T cell immunity. J Leukoc Biol 84:1238–1247
Kaiserman D, Bird PI (2010) Control of granzymes by serpins. Cell Death Differ 17:586–595
Khan MS, Singh P, Azhar A, Naseem A, Rashid Q, Kabir MA et al (2011) Serpin inhibition mechanism: a delicate balance between native metastable state and polymerization. J Amino Acids 2011:606797
Pike RN, Bottomley SP, Irving JA, Bird PI, Whisstock JC (2002) Serpins: finely balanced conformational traps. IUBMB Life 54:1–7
Izuhara K, Ohta S, Kanaji S, Shiraishi H, Arima K (2008) Recent progress in understanding the diversity of the human ov-serpin/clade B serpin family. Cell Mol Life Sci 65:2541–2553
Huntington JA (2006) Shape-shifting serpins—advantages of a mobile mechanism. Trends Biochem Sci 31:427–435
Olson ST, Bock PE, Kvassman J, Shore JD, Lawrence DA, Ginsburg et al (1995) Role of the catalytic serine in the interactions of serine proteinases with protein inhibitors of the serpin family. Contribution of a covalent interaction to the binding energy of serpin–proteinase complexes. J Biol Chem 270:30007–30017
Hunt LT, Dayhoff MO (1980) A surprising new protein superfamily containing ovalbumin, antithrombin-III, and alpha 1-proteinase inhibitor. Biochem Biophys Res Commun 95:864–871
Van Gent D, Sharp P, Morgan K, Kalsheker N (2003) Serpins: structure, function and molecular evolution. Int J Biochem Cell Biol 35:1536–1547
Benarafa C, Remold-O’Donnell E (2005) The ovalbumin serpins revisited: perspective from the chicken genome of clade B serpin evolution in vertebrates. Proc Natl Acad Sci USA 102:11367–11372
Scott FL, Eyre HJ, Lioumi M, Ragoussis J, Irving JA, Sutherland GA et al (1999) Human ovalbumin serpin evolution: phylogenic analysis, gene organization, and identification of new PI8-related genes suggest that two interchromosomal and several intrachromosomal duplications generated the gene clusters at 18q21–q23 and 6p25. Genomics 62:490–499
Janciauskiene S (2001) Conformational properties of serine proteinase inhibitors (serpins) confer multiple pathophysiological roles. Biochim Biophys Acta 1535:221–235
Remold-O’Donnell. Involvement of SerpinB1 in generation of NETs (neutrophil extracellular traps). The 6th International Symposium of the Chemistry and Biology of Serpins, Chapel Hill, NC, USA, 23–26 October 2011 (abstract).
Medcalf RL, Stasinopoulos SJ (2005) The undecided serpin. The ins and outs of plasminogen activator inhibitor type 2. FEBS J 272:4858–4867
Inagi R, Nangaku M, Miyata T, Kurokawa K (2003) Mesangial cell-predominant functional gene, megsin. Clin Exp Nephrol 7:87–92
Wang J, Yang L, Li J, Rezaie R. The cardioprotective activity of antithrombin through interaction with vascular HSPGs. The 6th International Symposium of the Chemistry and Biology of Serpins, Chapel Hill, NC, USA, 23–26 October 2011 (abstract).
Tollefsen DM. Heparin cofactor II modulates the response to arterial injury. The 6th International Symposium of the Chemistry and Biology of Serpins, Chapel Hill, NC, USA, 23–26 October 2011 (abstract).
Andreasen PA. PAI-1 in cancer. The 6th International Symposium of the Chemistry and Biology of Serpins, Chapel Hill, NC, USA, 23–26 October 2011 (abstract).
Selbonne S, Azibani F, Iatmanen S, Boulaftali Y, Jandrot-Perrus M, Bouton MC, et al. In vitro and in vivo anti-angiogenic properties of the tissue serpin protease nexin-1. The 6th International Symposium of the Chemistry and Biology of Serpins, Chapel Hill, NC, USA, 23–26 October 2011 (abstract)
Bouton MC. Emerging role of PN-1 in thrombosis and vascular biology. The 6th International Symposium of the Chemistry and Biology of Serpins, Chapel Hill, NC, USA, 23–26 October 2011 (abstract).
Fitzgerald DP, Subramanian P, Deshpande M, Graves C, Gordon I, Qian Y, et al. PEDF: opposing effects of metastatic cells and neurons in the brain. The 6th International Symposium of the Chemistry and Biology of Serpins, Chapel Hill, NC, USA, 23–26 October 2011 (abstract).
Becerra SP, Deshpande MM, Locatelli-Hoops S, Moghaddam-Taaheri S, Guerrier S, Balko N, et al. Identification of pigment epithelium-derived factor (PEDF) protein versions with distinct activities on tumor cells lines. The 6th International Symposium of the Chemistry and Biology of Serpins, Chapel Hill, NC, USA, 23–26 October 2011 (abstract).
Yepes M. Tissue-type plasminogen activator and neuroserpin in the central nervous system. The 6th International Symposium of the Chemistry and Biology of Serpins, Chapel Hill, NC, USA, 23–26 October 2011 (abstract).
Lee TW, Montgomery J, Christie DL, Birch NP. Neuroserpin and neural development: modulation of growth and morphological characteristics. The 6th International Symposium of the Chemistry and Biology of Serpins, Chapel Hill, NC, USA, 23–26 October 2011 (abstract).
Valiente M, Massague J. Neuroserpin mediates brain metastasis. The 6th International Symposium of the Chemistry and Biology of Serpins, Chapel Hill, NC, USA, 23–26 October 2011 (abstract).
Higgins WJ, Grehan G, Alhayek S, Worrall DM. Characterisation of human serpin I2; a protective role against prematurely activated pancreatic zymogens? The 6th International Symposium of the Chemistry and Biology of Serpins, Chapel Hill, NC, USA, 23–26 October 2011 (abstract).
Huntington JA, Sendall TJ, Yamasaki M (2009) New insight into serpin polymerization and aggregation. Prion 3:12–14
Yamasaki M, Li W, Johnson DJ, Huntington JA (2008) Crystal structure of a stable dimer reveals the molecular basis of serpin polymerization. Nature 455:1255–1258
Whisstock JC, Bottomley SP (2006) Molecular gymnastics: serpin structure, folding and misfolding. Curr Opin Struct Biol 16:761–768
Gooptu B, Lomas DA (2008) Polymers and inflammation: disease mechanisms of the serpinopathies. J Exp Med 205:1529–1534
Gooptu B, Lomas DA (2009) Conformational pathology of the serpins: themes, variations, and therapeutic strategies. Annu Rev Biochem 78:147–176
Devies MJ, Lomas DA (2008) The molecular aetiology of the serpinopathies. Int J Biochem Cell Biol 40:1273–1286
Lomas DA, Mahadeva R (2002) Alpha1-antitrypsin polymerization and the serpinopathies: pathobiology and prospects for therapy. J Clin Invest 110:1585–1590
Miyata T, Inagi R, Sugiyama S, Usuda N (2005) Serpinopathy and endoplasmic reticulum stress. Med Mol Morphol 38:73–78
Roussel BD, Irving JA, Ekeowa UI, Belorgey D, Haq I, Ordóñez A et al (2011) Unravelling the twists and turns of the serpinopathies. FEBS J 278:3859–3867
Inagi R, Nangaku M, Usuda N, Shimizu A, Onogi H, Izuhara Y et al (2005) Novel serpinopathy in rat kidney and pancreas induced by overexpression of megsin. J Am Soc Nephrol 16:1339–1349
Remold-O’Donnell E (1993) The ovalbumin family of serpin proteins. FEBS Lett 315:105–108
Kaiserman D, Bird PI (2005) Analysis of vertebrate genomes suggests a new model for clade B serpin evolution. BMC Genomics 6:167
Kaiserman D, Knaggs S, Scarff KL, Gillard A, Mirza G, Cadman M et al (2002) Comparison of human chromosome 6p25 with mouse chromosome 13 reveals a greatly expanded ov-serpin gene repertoire in the mouse. Genomics 79:349–362
Hirst CE, Buzza MS, Bird CH, Warren HS, Cameron PU, Zhang M et al (2003) The intracellular granzyme B inhibitor, proteinase inhibitor 9, is up-regulated during accessory cell maturation and effector cell degranulation, and its overexpression enhances CTL potency. J Immunol 170:805–815
Zhang YQ, Li P, Hou M, Wang X, Fan J, Tan L et al (2003) Identification of interaction between PAI-2 and IRF-3. Sheng Wu Hua Xue Yu Sheng Wu Wu Li Xue Bao (Shanghai) 35:661-5.
Palmiter RD, Gagnon J, Walsh KA (1978) Ovalbumin: a secreted protein without a transient hydrophobic leader sequence. Proc Natl Acad Sci U S A 75:94–98
Belin D (1993) Biology and facultative secretion of plasminogen activator inhibitor-2. Thromb Haemost 70:144–147
Pemberton PA, Tipton AR, Pavloff N, Smith J, Erickson JR, Mouchabeck ZM et al (1997) Maspin is an intracellular serpin that partitions into secretory vesicles and is present at the cell surface. J Histochem Cytochem 45:1697–1706
Von Heijne G, Liljeström P, Mikus P, Andersson H, Ny T (1991) The efficiency of the uncleaved secretion signal in the plasminogen activator inhibitor type 2 protein can be enhanced by point mutations that increase its hydrophobicity. J Biol Chem 266:15240–15243
Ritchie H, Booth NA (1998) Secretion of plasminogen activator inhibitor 2 by human peripheral blood monocytes occurs via an endoplasmic reticulum–Golgi-independent pathway. Exp Cell Res 242:439–450
Irving JA, Pike RN, Dai W, Brömme D, Worrall DM, Silverman GA et al (2002) Evidence that serpin architecture intrinsically supports papain-like cysteine protease inhibition: engineering alpha(1)-antitrypsin to inhibit cathepsin proteases. Biochemistry 41:4998–5004
Bird PI (1999) Regulation of pro-apoptotic leucocyte granule serine proteinases by intracellular serpins. Immunol Cell Biol 77:47–57
Kummer JA, Micheau O, Schneider P, Bovenschen N, Broekhuizen R, Quadir R et al (2007) Ectopic expression of the serine protease inhibitor PI9 modulates death receptor-mediated apoptosis. Cell Death Differ 14:1486–1496
Medema JP, Schuurhuis DH, Rea D, van Tongeren J, de Jong J, Bres SA et al (2001) Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: differential modulation by T helper type 1 and type 2 cells. J Exp Med 194:657–667
Missen MA, Haylock D, Whitty G, Medcalf RL, Coughlin PB (2006) Stage specific gene expression of serpins and their cognate proteases during myeloid differentiation. Br J Haematol 135:715–724
Bots M, Van Bostelen L, Rademaker MT, Offringa R, Medema JP (2006) Serpins prevent granzyme-induced death in a species-specific manner. Immunol Cell Biol 84:79–86
Ciscato F, Sciacovelli M, Ruvoletto MG, Quarta S, Turato C, Gatta A, et al. SERPINB3 inhibits the mitochondrial permeability transition pore. The 6th International Symposium of the Chemistry and Biology of Serpins, Chapel Hill, NC, USA, 23–26 October 2011 (abstract).
Ullman E, Pan JA, Zong WX (2011) Squamous cell carcinoma antigen 1 promotes caspase-8-mediated apoptosis in response to endoplasmic reticulum stress while inhibiting necrosis induced by lysosomal injury. Mol Cell Biol 31:2902–2919
Pontisso P, Calabrese F, Benvegnù L, Lise M, Belluco C, Ruvoletto MG et al (2004) Overexpression of squamous cell carcinoma antigen variants in hepatocellular carcinoma. Br J Cancer 90:833–837
Suminami Y, Nagashima S, Vujanovic NL, Hirabayashi K, Kato H, Whiteside TL (2000) Inhibition of apoptosis in human tumour cells by the tumour-associated serpin, SCC antigen-1. Br J Cancer 82:981–989
Numa F, Takeda O, Nakata M, Nawata S, Tsunaga N, Hirabayashi K et al (1996) Tumor necrosis factor-alpha stimulates the production of squamous cell carcinoma antigen in normal squamous cells. Tumour Biol 17:97–101
Vidalino L, Doria A, Quarta SM, Crescenzi M, Ruvoletto M, Frezzato F et al (2012) SERPINB3 expression on B-cell surface in autoimmune diseases and hepatitis C virus-related chronic liver infection. Exp Biol Med (Maywood) 237:793–803
Vidalino L, Doria A, Quarta S, Zen M, Gatta A, Pontisso P (2009) SERPINB3, apoptosis and autoimmunity. Autoimmun Rev 9:108–112
Uemura Y, Pak SC, Luke C, Cataltepe S, Tsu C, Schick C et al (2000) Circulating serpin tumor markers SCCA1 and SCCA2 are not actively secreted but reside in the cytosol of squamous carcinoma cells. Int J Cancer 89:368–377
Catanzaro JM, Guerriero JL, Liu J, Ullman E, Sheshadri N, Chen JJ et al (2011) Elevated expression of squamous cell carcinoma antigen (SCCA) is associated with human breast carcinoma. PLoS One 6:e19096
Song KJ, Ahn HJ, Nam HW (2012) Anti-apoptotic effects of SERPIN B3 and B4 via STAT6 activation in macrophages after infection with Toxoplasma gondii. Korean J Parasitol 50:1–6
Pontisso P, Morsica G, Ruvoletto MG, Zambello R, Colletta C, Chemello L et al (1991) Hepatitis B virus binds to peripheral blood mononuclear cells via the pre S1 protein. J Hepatol 12:203–206
Pontisso P, Vidalino L, Quarta S, Gatta A (2008) Biological and clinical implications of HBV infection in peripheral blood mononuclear cells. Autoimmun Rev 8:13–17
Hamanaka S, Ujihara M, Numa F, Kato H (1997) Serum level of squamous cell carcinoma antigen as a new indicator of disease activity in patients with psoriasis. Arch Dermatol 133:393–395
Giannelli G, Iannone F, Fransvea E, Chialà A, Lapadula G, Antonaci S (2007) Squamous cellular carcinoma immunocomplexed is increased in scleroderma patients with lung fibrosis. Clin Exp Rheumatol 25:794–795
El-Rachkidy RG, Young HS, Griffiths CE, Camp RD (2008) Humoral autoimmune responses to the squamous cell carcinoma antigen protein family in psoriasis. J Invest Dermatol 128:2219–2224
Khan S, Tarzi MD, Doré PC, Sewell WA, Longhurst HJ (2007) Secondary systemic lupus erythematosus: an analysis of 4 cases of uncontrolled hereditary angioedema. Clin Immunol 123:14–17
Brickman CM, Tsokos GC, Chused TM, Balow JE, Lawley TJ, Santaella M et al (1986) Immunoregulatory disorders associated with hereditary angioedema. II. Serologic and cellular abnormalities. J Allergy Clin Immunol 77:758–767
Cacoub P, Frémeaux-Bacchi V, De Lacroix I, Guillien F, Kahn MF, Kazatchkine MD et al (2001) A new type of acquired C1 inhibitor deficiency associated with systemic lupus erythematosus. Arthritis Rheum 44:1836–1840
Nettis E, Colanardi MC, Loria MP, Vacca A (2005) Acquired C1-inhibitor deficiency in a patient with systemic lupus erythematosus: a case report and review of the literature. Eur J Clin Invest 35:781–784
Lahiri M, Lim AY (2007) Angioedema and systemic lupus erythematosus—a complementary association? Ann Acad Med Singapore 36:142–145
Czyzyk J, Henegariu O, Preston-Hurlburt P, Baldzizhar R, Fedorchuk C, Esplugues E et al (2012) Enhanced anti-serpin antibody activity inhibits autoimmune inflammation in type 1 diabetes. J Immunol 188:6319–6327
Grimstein C, Choi YK, Wasserfall CH, Satoh M, Atkinson MA, Brantly ML et al (2011) Alpha-1 antitrypsin protein and gene therapies decrease autoimmunity and delay arthritis development in mouse model. J Transl Med 9:21
Sardi F, Fassina L, Venturini L, Inguscio M, Guerriero F, Rolfo E et al (2011) Alzheimer’s disease, autoimmunity and inflammation. The good, the bad and the ugly. Autoimmun Rev 11:149–153
Costa CZ, da Rosa SE, de Camargo MM (2011) The unfolded protein response: how protein folding became a restrictive aspect for innate immunity and B lymphocytes. Scand J Immunol 73:436–448
Rasheva VI, Domingos PM (2009) Cellular responses to endoplasmic reticulum stress and apoptosis. Apoptosis 14:996–1007
Inagi R (2009) Endoplasmic reticulum stress in the kidney as a novel mediator of kidney injury. Nephron Exp Nephrol 112:e1–e9
Vitadello M, Doria A, Tarricone E, Ghirardello A, Gorza L (2010) Myofiber stress-response in myositis: parallel investigations on patients and experimental animal models of muscle regeneration and systemic inflammation. Arthritis Res Ther 12(2):R52
Ekeowa UI, Freeke J, Miranda E, Gooptu B, Bush MF, Pérez J et al (2010) Defining the mechanism of polymerization in the serpinopathies. Proc Natl Acad Sci U S A 107:17146–17151
Lawless MV, Greene CM, Mulgrew A, Taggart CC, O’Neill SJ, McElvaney NG (2004) Activation of endoplasmic reticulum-specific stress responses associated with the conformational disease Z alpha 1-antitrypsin deficiency. J Immunol 172:5722–5760
Kaufman RJ (1999) Stress signaling from the lumen of the endoplasmic reticulum: coordination of gene transcriptional and translational controls. Genes Dev 13:1211–1233
Kroeger H, Miranda E, MacLeod I, Pérez J, Crowther DC, Marciniak SJ et al (2009) Endoplasmic reticulum-associated degradation (ERAD) and autophagy cooperate to degrade polymerogenic mutant serpins. J Biol Chem 284:22793–22802
Ishida Y, Nagata K (2009) Autophagy eliminates a specific species of misfolded procollagen and plays a protective role in cell survival against ER stress. Autophagy 5:1217–1219
Davies MJ, Miranda E, Roussel BD, Kaufman RJ, Marciniak SJ, Lomas DA (2009) Neuroserpin polymers activate NF-kappaB by a calcium signaling pathway that is independent of the unfolded protein response. J Biol Chem 284:18202–18209
Singh RP, Waldron RT, Hahn BH (2012) Genes, tolerance and systemic autoimmunity. Autoimmun Rev 11:664–669
Varin MM, Guerrier T, Devauchelle-Pensec V, Jamin C, Youinou P, Pers JO (2012) In Sjögren’s syndrome, B lymphocytes induce epithelial cells of salivary glands into apoptosis through protein kinase C delta activation. Autoimmun Rev 11:252–258
Iaccarino L, Ghirardello A, Canova M, Zen M, Bettio S, Nalotto L et al (2011) Anti-annexins autoantibodies: their role as biomarkers of autoimmune diseases. Autoimmun Rev 10:553–558
Comi C, Fleetwood T, Dianzani U (2012) The role of T cell apoptosis in nervous system autoimmunity. Autoimmun Rev 12:150–156
Zen M, Canova M, Campana C, Bettio S, Nalotto L, Rampudda M et al (2011) The kaleidoscope of glucorticoid effects on immune system. Autoimmun Rev 10:305–310
Racanelli V, Prete M, Musaraj G, Dammacco F, Perosa F (2011) Autoantibodies to intracellular antigens: generation and pathogenetic role. Autoimmun Rev 10:503–508
Costenbader KH, Gay S, Alarcón-Riquelme ME, Iaccarino L, Doria A (2012) Genes, epigenetic regulation and environmental factors: which is the most relevant in developing autoimmune diseases? Autoimmun Rev 11:604–609
Rekvig OP, Putterman C, Casu C, Gao HX, Ghirardello A, Mortensen ES et al (2012) Autoantibodies in lupus: culprits or passive bystanders? Autoimmun Rev 11:596–603
Münz C (2012) Antigen processing for MHC Class II presentation via autophagy. Front Immunol 3:9
Münz C (2010) Antigen processing via autophagy—not only for MHC class II presentation anymore? Curr Opin Immunol 22:89–93
Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L (2008) Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature 455:396–400
Deretic V (2009) Multiple regulatory and effector roles of autophagy in immunity. Curr Opin Immunol 21:53–62
Pierdominici R, Vomero M, Barbati C, Colasanti T, Maselli A, Vacirca D et al (2012) Role of autophagy in immunity and autoimmunity, with a special focus on systemic lupus erythematosus. FASEB J 26:1400–1412
Rodríguez-Bayona B, Ramos-Amaya A, Pérez-Venegas JJ, Rodríguez C, Brieva JA (2010) Decreased frequency and activated phenotype of blood CD27 IgD IgM B lymphocytes is a permanent abnormality in systemic lupus erythematosus patients. Arthritis Res Ther 12:R108
Hostmann A, Jacobi AM, Mei H, Hiepe F, Dörner T (2008) Peripheral B cell abnormalities and disease activity in systemic lupus erythematosus. Lupus 17:1064–1069
Korganow AS, Knapp AM, Nehme-Schuster H, Soulas-Sprauel P, Poindron V, Pasquali JL et al (2010) Peripheral B cell abnormalities in patients with systemic lupus erythematosus in quiescent phase: decreased memory B cells and membrane CD19 expression. J Autoimm 34:426–434
Shapira E, Proscura E, Brodsky B, Wormser U (2011) Novel peptides as potential treatment of systemic lupus erythematosus. Lupus 20:463–472
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gatto, M., Iaccarino, L., Ghirardello, A. et al. Serpins, Immunity and Autoimmunity: Old Molecules, New Functions. Clinic Rev Allerg Immunol 45, 267–280 (2013). https://doi.org/10.1007/s12016-013-8353-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12016-013-8353-3