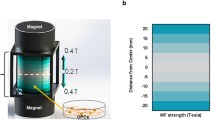
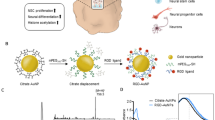
Abstract
Neurodevelopmental processes of pluripotent cells, such as proliferation and differentiation, are influenced by external natural forces. Despite the presence of biogenic magnetite nanoparticles in the central nervous system and constant exposure to the Earth’s magnetic fields and other sources, there is scant knowledge regarding the role of electromagnetic stimuli in neurogenesis. Moreover, emerging applications of electrical and magnetic stimulation to treat neurological disorders emphasize the relevance of understanding the impact and mechanisms behind these stimuli. Here, the effects of magnetic nanoparticles (MNPs) in polymeric coatings and the static external magnetic field (EMF) were investigated on neural induction of murine embryonic stem cells (mESCs) and human induced pluripotent stem cells (hiPSCs). The results show that the presence of 0.5% MNPs in collagen-based coatings facilitates the migration and neuronal maturation of mESCs and hiPSCs in vitro. Furthermore, the application of 0.4 Tesla EMF perpendicularly to the cell culture plane, discernibly stimulates proliferation and guide fate decisions of the pluripotent stem cells, depending on the origin of stem cells and their developmental stage. Mechanistic analysis reveals that modulation of ionic homeostasis and the expression of proteins involved in cytostructural, liposomal and cell cycle checkpoint functions provide a principal underpinning for the impact of electromagnetic stimuli on neural lineage specification and proliferation. These findings not only explore the potential of the magnetic stimuli as neural differentiation and function modulator but also highlight the risks that immoderate magnetic stimulation may affect more susceptible neurons, such as dopaminergic neurons.
Graphical Abstract







Similar content being viewed by others
References
Erdmann, W., Kmita, H., Kosicki, J. Z., & Kaczmarek, Ł. (2021). How the Geomagnetic Field Influences Life on Earth - An Integrated Approach to Geomagnetobiology. Origins of Life and Evolution of the Biosphere: The Journal of the International Society for the Study of the Origin of Life, 51(3), 231–257. https://doi.org/10.1007/s11084-021-09612-5
Buis, A. (2021). [online] Available at: https://climate.nasa.gov/news/3105/earths-magnetosphere-protecting-our-planet-from-harmful-space-energy NASA's Jet Propulsion Laboratory, 2021. [Accessed 21 Nov. 2021].
Kirschvink, J. L., Kobayashi-kirschvink, A., & Woodford, B. J. (1992). Magnetite biomineralization in the human brain, 89(August), 7683–7687.
Kenneth, J. (2010). Magnetic-field perception. Nature Q&A, 464(22), 1140–1142.
Størmer, F. C., Mysterud, I., & Slagsvold, T. (2011). Evolution and possible storage of information in a magnetite system of significance for brain development. Medical Hypotheses, 76(6), 901–904. https://doi.org/10.1016/j.mehy.2011.03.004
Gieré, R. (2016). Magnetite in the human body: Biogenic vs. anthropogenic. Proceedings of the National Academy of Sciences, 113(43), 11986–11987. https://doi.org/10.1073/pnas.1613349113
Dobson, J. (2002). Investigation of age-related variations in biogenic magnetite levels in the human hippocampus. Experimental Brain Research, 144(1), 122–126. https://doi.org/10.1007/s00221-002-1066-0
Brooks, J., Everett, J., Lermyte, F., Tjendana Tjhin, V., Sadler, P. J., Telling, N., & Collingwood, J. F. (2020). Analysis of neuronal iron deposits in Parkinson’s disease brain tissue by synchrotron x-ray spectromicroscopy. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements (GMS), 62, 126555. https://doi.org/10.1016/j.jtemb.2020.126555
Mo, W. chuan, Zhang, Z. jian, Liu, Y., Bartlett, P. F., & He, R. qiao. (2013). Magnetic Shielding Accelerates the Proliferation of Human Neuroblastoma Cell by Promoting G1-Phase Progression. PLoS One, 8(1), 1–11. https://doi.org/10.1371/journal.pone.0054775.
Semeano, A. T., Glaser, T., Ulrich, H., & Petri, D. F. S. P. (2016). Scaffolds for Embryonic Stem Cell Growth and Differentiation. In Working with Stem Cells (pp. 347–365).
Pacini, S., Vannelli, G. B., Barni, T., Ruggiero, M., Sardi, I., Pacini, P., & Gulisano, M. (1999). Effect of 0.2 T static magnetic field on human neurons: Remodeling and inhibition of signal transduction without genome instability. Neuroscience Letters, 267(3), 185–188. https://doi.org/10.1016/S0304-3940(99)00362-6
Kaebisch, C., Schipper, D., Babczyk, P., & Tobiasch, E. (2015). The role of purinergic receptors in stem cell differentiation. Computational and Structural Biotechnology Journal, 13, 75–84. https://doi.org/10.1016/j.csbj.2014.11.003
Tabar, V., & Studer, L. (2014). Pluripotent stem cells in regenerative medicine : challenges and recent progress. Nature Publishing Group, 15(2), 82–92. https://doi.org/10.1038/nrg3563
Trounson, A., & Dewitt, N. D. (2016). Pluripotent stem cells progressing to the clinic st l S S e. Nature Publishing Group, 17(3), 194–200. https://doi.org/10.1038/nrm.2016.10
Krishna, L., Dhamodaran, K., Jayadev, C., Chatterjee, K., Shetty, R., & Khora, S. S. (2016). Nanostructured scaffold as a determinant of stem cell fate. Stem Cell Research & Therapy, 1–12. https://doi.org/10.1186/s13287-016-0440-y
da Silva, A. C., Semeano, A. T. S., Dourado, A. H. B., Ulrich, H., & Cordoba de Torresi, S. I. (2018). Novel Conducting and Biodegradable Copolymers with Noncytotoxic Properties toward Embryonic Stem Cells. ACS Omega, 3(5), 5593–5604. https://doi.org/10.1021/acsomega.8b00510
Fornazari, M., Nascimento, I. C., Nery, A. A., da Silva, C. C. C., Kowaltowski, A. J., & Ulrich, H. (2011). Neuronal differentiation involves a shift from glucose oxidation to fermentation. Journal of Bioenergetics and Biomembranes, 43(5), 531–539. https://doi.org/10.1007/s10863-011-9374-3
Glaser, T., de Oliveira, S. L. B., Cheffer, A., Beco, R., Martins, P., Fornazari, M., et al. (2014). Modulation of mouse embryonic stem cell proliferation and neural differentiation by the P2X7 receptor. PLoS One, 9(5), e96281. https://doi.org/10.1371/journal.pone.0096281
Tofoli, F. de A., Chien, H. F., Barbosa, E. R., & Pereira, L. V. (2019). Generation of 5 hiPSC lines derived from three unrelated idiopathic Parkinson disease patients and two unrelated healthy control individuals. Stem Cell Research, 41, 101640. https://doi.org/10.1016/j.scr.2019.101640
Tofoli, F. A., Semeano, A. T. S., Oliveira-Giacomelli, Á., Gonçalves, M. C. B., Ferrari, M. F. R., Veiga Pereira, L., & Ulrich, H. (2019). Midbrain Dopaminergic Neurons Differentiated from Human-Induced Pluripotent Stem Cells. Methods in Molecular Biology (Clifton, N.J.), 1919, 97–118. https://doi.org/10.1007/978-1-4939-9007-8_8.
Bueno, V. B., Silva, A. M., Barbosa, L. R. S., Catalani, L. H., Teixeira-Neto, É., Cornejo, D. R., & Petri, D. F. S. (2013). Hybrid composites of xanthan and magnetic nanoparticles for cellular uptake. Chemical Communications, 49(85), 9911–9913. https://doi.org/10.1039/C3CC42277A
Strober, W. (2015). Trypan Blue Exclusion Test of Cell Viability. Current Protocols in Immunology, 111, A3.B.1-A3.B.3. https://doi.org/10.1002/0471142735.ima03bs111.
Martins, A. H. B., Resende, R. R., Majumder, P., Faria, M., Casarini, D. E., Tárnok, A., et al. (2005). Neuronal differentiation of P19 embryonal carcinoma cells modulates kinin B2 receptor gene expression and function. The Journal of Biological Chemistry, 280(20), 19576–19586. https://doi.org/10.1074/jbc.M502513200
Kundu, A., Patel, A., & Pal, A. (2013). Defining reference genes for qPCR normalization to study biotic and abiotic stress responses in Vigna mungo. Plant Cell Reports, 32(10), 1647–1658. https://doi.org/10.1007/s00299-013-1478-2
Mootha, V. K., Lindgren, C. M., Eriksson, K.-F., Subramanian, A., Sihag, S., Lehar, J., et al. (2003). PGC-1alpha-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature Genetics, 34(3), 267–273. https://doi.org/10.1038/ng1180
Subramanian, A., Tamayo, P., Mootha, V. K., Mukherjee, S., Ebert, B. L., Gillette, M. A., … Mesirov, J. P. (2005). Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proceedings of the National Academy of Sciences, 102(43), 15545–15550. https://doi.org/10.1073/pnas.0506580102
Glaser, T., Shimojo, H., Ribeiro, D. E., Martins, P. P. L., Beco, R. P., Kosinski, M., et al. (2021). ATP and spontaneous calcium oscillations control neural stem cell fate determination in Huntington’s disease: a novel approach for cell clock research. Molecular Psychiatry, 26(6), 2633–2650. https://doi.org/10.1038/s41380-020-0717-5
Glaser, T., Andrejew, R., Oliveira-Giacomelli, Á., Ribeiro, D. E., Bonfim Marques, L., Ye, Q., et al. (2020). Purinergic Receptors in Basal Ganglia Diseases: Shared Molecular Mechanisms between Huntington’s and Parkinson’s Disease. Neuroscience Bulletin, 36(11), 1299–1314. https://doi.org/10.1007/s12264-020-00582-8
Flierl, A., Oliveira, L. M. A., Falomir-Lockhart, L. J., Mak, S. K., Hesley, J., Soldner, F., et al. (2014). Higher vulnerability and stress sensitivity of neuronal precursor cells carrying an alpha-synuclein gene triplication. PLoS One, 9(11), e112413. https://doi.org/10.1371/journal.pone.0112413
Viegas, A. D. C., Geshev, J., Schmidt, J. E., & Ferrari, E. F. (1998). Giant magnetoresistance and remanence in granular CoCu codeposited films. Journal of Applied Physics, 83(11), 7007–7009. https://doi.org/10.1063/1.367808
Glaser, T., Bueno, V. B., Cornejo, D. R., Petri, D. F. S., & Ulrich, H. (2015). Neuronal adhesion, proliferation and differentiation of embryonic stem cells on hybrid scaffolds made of xanthan and magnetite nanoparticles. Biomedical Materials (Bristol), 10(4). https://doi.org/10.1088/1748-6041/10/4/045002
Castro, P. S., Bertotti, M., Naves, A. F., Catalani, L. H., Cornejo, D. R., Bloisi, G. D., & Petri, D. F. S. (2017). Hybrid magnetic scaffolds: The role of scaffolds charge on the cell proliferation and Ca2+ ions permeation. Colloids and Surfaces B: Biointerfaces, 156, 388–396. https://doi.org/10.1016/j.colsurfb.2017.05.046
Singh, N., Jenkins, G. J. S., Asadi, R., & Doak, S. H. (2010). Potential toxicity of superparamagnetic iron oxide nanoparticles (SPION). Nano Reviews, 1. https://doi.org/10.3402/nano.v1i0.5358
Wahajuddin, null, & Arora, S. (2012). Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. International Journal of Nanomedicine, 7, 3445–3471. https://doi.org/10.2147/IJN.S30320
Yu, D., Neeley, W. L., Pritchard, C. D., Slotkin, J. R., Woodard, E. J., Langer, R., & Teng, Y. D. (2009). Blockade of peroxynitrite-induced neural stem cell death in the acutely injured spinal cord by drug-releasing polymer. Stem Cells, 27(5), 1212–1222. https://doi.org/10.1002/stem.26
Mounsey, R. B., & Teismann, P. (2012). Chelators in the treatment of iron accumulation in Parkinson’s disease. International journal of cell biology, 2012, 983245. https://doi.org/10.1155/2012/983245
Kim, S., Im, W. S., Kang, L., Lee, S. T., Chu, K., & Kim, B. I. (2008). The application of magnets directs the orientation of neurite outgrowth in cultured human neuronal cells. Journal of Neuroscience Methods, 174(1), 91–96. https://doi.org/10.1016/j.jneumeth.2008.07.005
Mo, W.-C., Zhang, Z.-J., Wang, D.-L., Liu, Y., Bartlett, P. F., & He, R.-Q. (2016). Shielding of the Geomagnetic Field Alters Actin Assembly and Inhibits Cell Motility in Human Neuroblastoma Cells. Scientific Reports, 6(1), 22624. https://doi.org/10.1038/srep22624
Wang, Z., Hao, F., Ding, C., Yang, Z., & Shang, P. (2014). Effects of static magnetic field on cell biomechanical property and membrane ultrastructure. Bioelectromagnetics, 35(4), 251–261. https://doi.org/10.1002/bem.21847
Wang, Z., Sarje, A., Che, P. L., & Yarema, K. J. (2009). Moderate strength (0.23-0.28 T) static magnetic fields (SMF) modulate signaling and differentiation in human embryonic cells. BMC Genomics, 10, 1–23. https://doi.org/10.1186/1471-2164-10-356
Wang, Z., Che, P.-L., Du, J., Ha, B., & Yarema, K. J. (2010). Static Magnetic Field Exposure Reproduces Cellular Effects of the Parkinson’s Disease Drug Candidate ZM241385. PLoS One, 5(11), e13883. https://doi.org/10.1371/journal.pone.0013883
Lew, W.-Z., Huang, Y.-C., Huang, K.-Y., Lin, C.-T., Tsai, M.-T., & Huang, H.-M. (2016). Static magnetic fields enhance dental pulp stem cell proliferation by activating the p38 mitogen-activated protein kinase pathway as its putative mechanism. Journal of Tissue Engineering and Regenerative Medicine, 12(1), 19–29. https://doi.org/10.1002/term.2333
Papke, D., Gonzalez-Gutierrez, G., & Grosman, C. (2011). Desensitization of neurotransmitter-gated ion channels during high-frequency stimulation: A comparative study of Cys-loop, AMPA and purinergic receptors. Journal of Physiology, 589(7), 1571–1585. https://doi.org/10.1113/jphysiol.2010.203315
Hao, J., & Delmas, P. (2010). Multiple desensitization mechanisms of mechanotransducer channels shape firing of mechanosensory neurons. Journal of Neuroscience, 30(40), 13384–13395. https://doi.org/10.1523/JNEUROSCI.2926-10.2010
Graczyk, E. L., Delhaye3, B. P., Schiefer, M. A., Bensmaia, S. J., & Tyler, D. J. (2019). Sensory adaptation to electrical stimulation of the somatosensory nerves. Journal of Neural Engineering, 15(4), 1–15. https://doi.org/10.1088/1741-2552/aab790.Sensory.
Li, H., Mapolelo, D. T., Randeniya, S., Johnson, M. K., & Outten, C. E. (2012). Human glutaredoxin 3 forms [2Fe-2S]-bridged complexes with human BolA2. Biochemistry, 51(8), 1687–1696. https://doi.org/10.1021/bi2019089.
Banci, L., Camponeschi, F., Ciofi-Baffoni, S., & Muzzioli, R. (2015). Elucidating the Molecular Function of Human BOLA2 in GRX3-Dependent Anamorsin Maturation Pathway. Journal of the American Chemical Society, 137(51), 16133–16143. https://doi.org/10.1021/jacs.5b10592
Frey, A. G., Palenchar, D. J., Wildemann, J. D., & Philpott, C. C. (2016). A Glutaredoxin·BolA Complex Serves as an Iron-Sulfur Cluster Chaperone for the Cytosolic Cluster Assembly Machinery. The Journal of Biological Chemistry, 291(43), 22344–22356. https://doi.org/10.1074/jbc.M116.744946
Nuttle, X., Giannuzzi, G., Duyzend, M. H., Schraiber, J. G., Narvaiza, I., Sudmant, P. H., et al. (2016). Emergence of a Homo sapiens-specific gene family and chromosome 16p11.2 CNV susceptibility. Nature, 536(7615), 205–209. https://doi.org/10.1038/nature19075
Ghiani, C. A., Starcevic, M., Rodriguez-Fernandez, I. A., Nazarian, R., Cheli, V. T., Chan, L. N., … Dell’Angelica, E. C. (2010). The dysbindin-containing complex (BLOC-1) in brain: developmental regulation, interaction with SNARE proteins and role in neurite outgrowth. Molecular Psychiatry, 15(2), 115, 204–215. https://doi.org/10.1038/mp.2009.58.
Fraldi, A., Klein, A. D., Medina, D. L., & Settembre, C. (2016). Brain Disorders Due to Lysosomal Dysfunction. Annual Review of Neuroscience, 39, 277–295. https://doi.org/10.1146/annurev-neuro-070815-014031
Fivenson, E. M., Lautrup, S., Sun, N., Scheibye-Knudsen, M., Stevnsner, T., Nilsen, H., et al. (2017). Mitophagy in neurodegeneration and aging. Neurochemistry International, 109, 202–209. https://doi.org/10.1016/j.neuint.2017.02.007
Okarmus, J., Bogetofte, H., Schmidt, S. I., Ryding, M., García-López, S., Ryan, B. J., et al. (2020). Lysosomal perturbations in human dopaminergic neurons derived from induced pluripotent stem cells with PARK2 mutation. Scientific Reports, 10(1), 1–16. https://doi.org/10.1038/s41598-020-67091-6
Demers-Lamarche, J., Guillebaud, G., Tlili, M., Todkar, K., Bélanger, N., Grondin, M., et al. (2016). Loss of mitochondrial function impairs Lysosomes∗. Journal of Biological Chemistry, 291(19), 10263–10276. https://doi.org/10.1074/jbc.M115.695825
Bergmann, F., & Keller, B. U. (2004). Impact of mitochondrial inhibition on excitability and cytosolic Ca2+ levels in brainstem motoneurones from mouse. The Journal of Physiology, 555(Pt 1), 45–59. https://doi.org/10.1113/jphysiol.2003.053900
Liu, Y., & Zhang, Y. (2019). ETV5 is Essential for Neuronal Differentiation of Human Neural Progenitor Cells by Repressing NEUROG2 Expression. Stem Cell Reviews and Reports, 15(5), 703–716. https://doi.org/10.1007/s12015-019-09904-4.
Adami, R., & Bottai, D. (2019). Spinal Muscular Atrophy Modeling and Treatment Advances by Induced Pluripotent Stem Cells Studies. Stem Cell Reviews and Reports, 15(6), 795–813. https://doi.org/10.1007/s12015-019-09910-6
Hansena, S. M., Berezinb, V., & Bockb, E. (2008). Signaling mechanisms of neurite outgrowth induced by the cell adhesion molecules NCAM and N-Cadherin. Cellular and Molecular Life Sciences, 65, 3809–3821. https://doi.org/10.1007/s00018-008-8290-0
Ghashghaei, H. T., Lai, C., & Anton, E. S. (2007). Neuronal migration in the adult brain : are we there yet ?, 8(February), 141–151. https://doi.org/10.1038/nrn2074.
Zablotskii, V., Polyakova, T., Lunov, O., & Dejneka, A. (2016). How a High-Gradient Magnetic Field Could Affect Cell Life. Scientific Reports, 6, 37407. https://doi.org/10.1038/srep37407
Kirschvink, J. L. (1989). Magnetite biomineralization and geomagnetic sensitivity in higher animals: an update and recommendations for future study. Bioelectromagnetics, 10(3), 239–259. https://doi.org/10.1002/bem.2250100304
Yarjanli, Z., Ghaedi, K., Esmaeili, A., Rahgozar, S., & Zarrabi, A. (2017). Iron oxide nanoparticles may damage to the neural tissue through iron accumulation, oxidative stress, and protein aggregation. BMC Neuroscience, 18(1), 1–12. https://doi.org/10.1186/s12868-017-0369-9
Goldman, S. (2003). Glia as neural progenitor cells. Trends in Neurosciences, 26(11), 590–596. https://doi.org/10.1016/j.tins.2003.09.011
Luarte, A., Cisternas, P., Caviedes, A., Batiz, L. F., Lafourcade, C., Wyneken, U., & Henzi, R. (2017). Astrocytes at the Hub of the Stress Response: Potential Modulation of Neurogenesis by miRNAs in Astrocyte-Derived Exosomes. Stem Cells International, 2017, 1719050. https://doi.org/10.1155/2017/1719050
Song, H., Stevens, C. F., & Gage, F. H. (2002). Astroglia induce neurogenesis from adult neural stem cells. Nature, 417(6884), 39–44. https://doi.org/10.1038/417039a
Salter, M. W., & Hicks, J. L. (1995). ATP causes release of intracellular Ca2+ via the phospholipase Cβ/IP3 pathway in astrocytes from the dorsal spinal cord. Journal of Neuroscience, 15(4), 2961–2971. https://doi.org/10.1523/jneurosci.15-04-02961.1995
Spitzer, N. C., Root, C. M., & Borodinsky, L. N. (2004). Orchestrating neuronal differentiation: Patterns of Ca2+ spikes specify transmitter choice. Trends in Neurosciences, 27(7), 415–421. https://doi.org/10.1016/j.tins.2004.05.003
Resende, R. R., Alves, A. S., Britto, L. R. G., & Ulrich, H. (2008). Role of acetylcholine receptors in proliferation and differentiation of P19 embryonal carcinoma cells. Experimental Cell Research, 314(7), 1429–1443. https://doi.org/10.1016/j.yexcr.2008.01.003
Sarichelou, I., Cappuccio, I., Ferranti, F., Mosillo, P., Ciceroni, C., Sale, P., et al. (2008). Metabotropic glutamate receptors regulate differentiation of embryonic stem cells into GABAergic neurons. Cell Death and Differentiation, 15(4), 700–707. https://doi.org/10.1038/sj.cdd.4402298
Giannuzzi, G., Schmidt, P. J., Porcu, E., Willemin, G., Munson, K. M., Nuttle, X., et al. (2019). The Human-Specific BOLA2 Duplication Modifies Iron Homeostasis and Anemia Predisposition in Chromosome 16p11.2 Autism Individuals. American Journal of Human Genetics, 105(5), 947–958. https://doi.org/10.1016/j.ajhg.2019.09.023
Acknowledgments
HU acknowledges grant support by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, proj. No. 2018/07366-4). DFSP acknowledges grant support by FAPESP (2018/134942-2). ATS is grateful for a doctorate fellowship granted by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, proj. No. 163310/2014-9). LVP acknowledges grant support FAPESP/CEPID 2013/08135-2. FTA is grateful for a doctorate fellowship granted by FAPESP (2014/25487-3), Conselho Nacional de Desenvolvimento Científico e Tecnológico Departamento de Ciência e Tecnologia do Ministério da Saúde (Neurodegenerative Diseases Study - chamada CNPq/MS/DECIT- 24/2014), Banco Nacional de Desenvolvimento Economico e Social (BNDES), Fundação de Apoio à Universidade de São Paulo (FUSP – project 2358) and Sanofi Genzyme Coorporation (Gaucher Generation 2010 grant program - GZ-2011-10731). MFRF acknowledges grant support by FAPESP (project No. 2013/08028-1; 2018/07592-4). RRC received a scholarship from FAPESP (project No. 2016/14513-8). JC-V was funded by a postdoctoral fellowship of the INCT-REGENERA (National Institute of Science and Technology in Regenerative Medicine). APJS is grateful for a doctorate fellowship and an exchange doctorate fellowship financed by Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brazil (CAPES, proj. No. 88882.332985/2018-01 and 8881.186506/2018-01). ÁO-G is grateful for a postdoctoral fellowship granted by FAPESP (2019/26852-0). Y.D.T. acknowledges funding by The US AMRMC (W81XWH-15-1-0621), The SCI Trust Fund of The Commonwealth of Massachusetts, The Gordon Project to Cure Clinical Paralysis, The Cele H. and William B. Rubin Family Fund, and The Roosevelt Warm Springs Foundation. The authors thank Prof. Daniel R. Cornejo, Institute of Physics, University of São Paulo, for the magnetization measurements. The graphical abstract and Fig. 1 were created using Biorender.com.
Availability of Data and Material
Data are available on request from Dr. Ana T. Semeano, Prof. Denise F.S. Petri and Prof. Henning Ulrich.
Author information
Authors and Affiliations
Contributions
ATSS, FTA, HU and DFSP conceived conceptual ideas; ATSS, FTA JC-V, APJS and RRC performed the experiments and data interpretation; ÁO-G, MAP, MFRF, ELR and GR performed data analysis; ATSS wrote the manuscript under supervision of HU and DSP; HU, DFSP, YDT, LVP, FTA, ÁO-G and MFRF made suggestions on manuscript, revised and edited the manuscript; HU, DFSP and LVP obtained financing of the project and supervised the experimental work.
Corresponding authors
Ethics declarations
Ethics’ Approval
The project was submitted to the Ethics Committee in Research of the University of Sao Paulo (USP-CEP) and approved for human research (Protocol: 112/2010) and for experiments with animals (Protocol: 116/2010). It was also approved by the Departmental Board of the Genetics Department and Evolutionary Biology of Biosciences Institute (BI) - USP (Order N°: 354–2011, Meeting: 399, Item N°: 3.2, Date: 02.03.2011; by the Ethics Committee for Analysis of Research Projects (CAPPesq) of the Hospital das Clínicas (N° 0754/11) by CEP Hemorio (Project No. 251/11,Date:03/15/2011); and by the Brazilian National Committee of Ethics in Research (CONEP) - 071/2013, registry n° 16.899, process No. 25000.069088–2012-57.
Consent for Publication
This manuscript has been approved by all authors and is solely the work of the authors named.
Conflict of Interest
H.U. obtains consulting fees from TissueGnostics, Vienna, Austria. This did not influence data acquisition and analysis and drawn conclusions. The other authors declare that they have no known competing financial interests or personal relationships that influence the work reported in this paper.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article belongs to the Topical Collection: Special Issue on Neurogenesis and Neurodegeneration: Basic Research and Clinic Applications
Guest Editor: Henning Ulrich
Supplementary Information

Table S1
Final concentrations of the reagents used to supplement dopaminergic neural differentiation medium (PNG 69 kb)

Table S2
Sequences of primers used in real-time PCR assays (PNG 65 kb)

Table S3
Primary antibodies used in immunocytochemistry (CI) and flow cytometry (CF) assays (PNG 63 kb)

Table S4
Secondary antibodies used in immunocytochemistry (CI) and flow cytometry (CF) assays (PNG 38 kb)

Table S5
Complementary characteristics of hiPSCs samples. The study was performed with 3 samples of peripheral blood mononuclear cells (PBMNC) collected and reprogrammed into iPSC (PNG 25 kb)

Fig. S1
Chronological representation of the culture and neuronal differentiation of embryonic stem cells. E14TG2a mouse embryonic stem cells (ESCs) were maintained in a pluripotency stage due to the presence of leukemia inhibitory factor (LIF) during (Step 0). After at least two passages in this stage, we proceeded to Step 1 with the formation of EBs in non-adherent culture for 2 days, followed by the induction of neural differentiation with retinoic acid (RA) for another 4 days. On the 6th day of differentiation, Step 2 begins. In this step 2, embryoid bodies (EBs) were seeded onto adherent polymeric substrates, promoting cell migration for 2 days, and neuronal differentiation was induced by culture medium supplementation with basic fibroblast growth factor (bFGF) and N-2 for another 12 days, with exchanging for fresh medium every 2 days. The representative images of each stage, presented in (A), (B) and (C) show, respectively, the colonies of undifferentiated ESCs (400μm calibration bar), suspended EBs (1050 μm calibration bar) and cell migration and differentiation from the adhered EBs (400 μm calibration bar) (PNG 274 kb)

Fig. S2
Chronological representation of the culture and dopaminergic neuronal differentiation. Undifferentiated human induced pluripotent stem cells (iPSCs) were grown in E8 medium until reaching 90% confluence, proceeding with daily medium refreshment containing 1x penicillin/ streptomycin antibiotics and 1x Glutamax and additionally supplemented with the factors schematically detailed in this timeline: cAMP: cyclic adenosine monophosphate; AA: Ascorbic Acid (vitamin C); BDNF: brain derived neurotrophic factor, CHIR: CHIR99021: DAPT: γ-secretase inhibitor; FGF-8: fibroblast growth factor 8; GDNF: glial cell-line derived neurotrophic factor; LDN: LDN193189; Pur.: purmorphamine; SB: SB431542; SHH: sonic hedgehog; TGFβ3: transforming growth factor beta 3. Respective concentrations dare detailed in Table S1. On day 20 of differentiation (DIV 20), cell passages of 2x105 cells/ well are performed into 12-well plates previously treated with 2x Geltrex coating. The cells were grown at 37 °C in KnockOut™ Serum Replacement (KRS) medium in a controlled atmosphere of 5% CO2 and 95% humidity. Representative images taken on DIV 2 ((A), 1,000 μm scale bar), DIV 20 ((B), 400 μm scale bar) and DIV 36 ((C), 400 μm scale bar) (PNG 407 kb)

Fig. S3
Influence of the polymeric substrate and the presence of MNPs on adhesion of EBs. EBs were seeded on gelatin, poly-L-lysine, xanthan and polymer blend CPAM: xanthan (1: 10) coating matrices, with and without 1 wt% MNPs in the polymeric substrate composition. Cell adhesion was quantified by the percentage of adhered EBs. Data are reported as mean values ± SD; * p < 0.05 and ** p < 0.001 for culture on substrates in the presence of MNPs compared to culture on their substrates in the absence of MNPs (control) (PNG 89 kb)

Fig. S4
Alignment of MNPs surrounding EBs and accumulating along neural processes. The red and white arrows show the orientation of MNPs along neural elongations and EBs, respectively (PNG 380 kb)

Fig. S5
Morphology of cell culture stimulated with EMF (N/S) during Stage I, Stage II or both (I=II). Scale bar 60 μm (PNG 297 kb)

Fig. S6
Illustrative images of cells labeled with LysoSensor blue-yellow. Acidic vesicles were labeled in yellow, and less acidic vesicles were blue. A-C control cells; D-E cells under EMF (PNG 148 kb)
Rights and permissions
About this article
Cite this article
Semeano, A.T., Tofoli, F.A., Corrêa-Velloso, J.C. et al. Effects of Magnetite Nanoparticles and Static Magnetic Field on Neural Differentiation of Pluripotent Stem Cells. Stem Cell Rev and Rep 18, 1337–1354 (2022). https://doi.org/10.1007/s12015-022-10332-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-022-10332-0