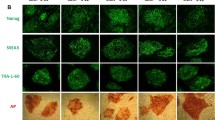
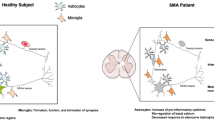
Abstract
Spinal Muscular Atrophy (SMA) is a neurodegenerative disease characterized by specific and predominantly lower motor neuron (MN) loss. SMA is the main reason for infant death, while about one in 40 children born is a healthy carrier. SMA is caused by decreased levels of production of a ubiquitously expressed gene: the survival motor neuron (SMN). All SMA patients present mutations of the telomeric SMN1 gene, but many copies of a centromeric, partially functional paralog gene, SMN2, can somewhat compensate for the SMN1 deficiency, scaling inversely with phenotypic harshness. Because the study of neural tissue in and from patients presents too many challenges and is very often not feasible; the use of animal models, such as the mouse, had a pivotal impact in our understanding of SMA pathology but could not portray totally satisfactorily the elaborate regulatory mechanisms that are present in higher animals, particularly in humans. And while recent therapeutic achievements have been substantial, especially for very young infants, some issues should be considered for the treatment of older patients. An alternative way to study SMA, and other neurological pathologies, is the use of induced pluripotent stem cells (iPSCs) derived from patients. In this work, we will present a wide analysis of the uses of iPSCs in SMA pathology, starting from basic science to their possible roles as therapeutic tools.


Similar content being viewed by others
References
Takahashi, K., & Yamanaka, S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell, 126(4), 663–676.
Dubowitz, V. (1974). Benign infantile spinal muscular atrophy. Developmental Medicine and Child Neurology, 16(5), 672–675.
Dubowitz, V. (1999). Very severe spinal muscular atrophy (SMA type 0): an expanding clinical phenotype. European Journal of Paediatric Neurology, 3(2), 49–51.
Pearn, J. H., Hudgson, P., & Walton, J. N. (1978). A clinical and genetic study of spinal muscular atrophy of adult onset: the autosomal recessive form as a discrete disease entity. Brain, 101(4), 591–606.
Bottai, D., & Adami, R. (2013). Spinal muscular atrophy: new findings for an old pathology. Brain Pathology, 23(6), 613–622.
Werdnig, G. (1891). Zwei frühinfantile hereditäre Fälle von progressiver Muskelatrophie unter dem Bilde der Dystrophie, aber anf neurotischer Grundlage. Archiv für Psychiatrie und Nervenkrankheiten, 22(2), 437–480.
Werdnig, G. (1971). Two early infantile hereditary cases of progressive muscular atrophy simulating dystrophy, but on a neural basis. 1891. Archives of Neurology, 25(3), 276–278.
Sugarman, E. A., Nagan, N., Zhu, H., Akmaev, V. R., Zhou, Z., Rohlfs, E. M., Flynn, K., Hendrickson, B. C., Scholl, T., Sirko-Osadsa, D. A., & Allitto, B. A. (2012). Pan-ethnic carrier screening and prenatal diagnosis for spinal muscular atrophy: clinical laboratory analysis of >72 400 specimens. European Journal of Human Genetics, 20(1), 27–32.
Prior, T. W., & Russman, B. S. (1993). Spinal Muscular Atrophy. Seattle (WA): University of Washington.
Rudnik-Schoneborn, S., Forkert, R., Hahnen, E., Wirth, B., & Zerres, K. (1996). Clinical spectrum and diagnostic criteria of infantile spinal muscular atrophy: further delineation on the basis of SMN gene deletion findings. Neuropediatrics, 27(1), 8–15.
Rudnik-Schoneborn, S., Hausmanowa-Petrusewicz, I., Borkowska, J., & Zerres, K. (2001). The predictive value of achieved motor milestones assessed in 441 patients with infantile spinal muscular atrophy types II and III. European Neurology, 45(3), 174–181.
Mercuri, E., Bertini, E., & Iannaccone, S. T. (2012). Childhood spinal muscular atrophy: controversies and challenges. Lancet Neurology, 11(5), 443–452.
Finkel, R. S., McDermott, M. P., Kaufmann, P., Darras, B. T., Chung, W. K., Sproule, D. M., Kang, P. B., Foley, A. R., Yang, M. L., Martens, W. B., Oskoui, M., Glanzman, A. M., Flickinger, J., Montes, J., Dunaway, S., O'Hagen, J., Quigley, J., Riley, S., Benton, M., Ryan, P. A., Montgomery, M., Marra, J., Gooch, C., & De Vivo, D. C. (2014). Observational study of spinal muscular atrophy type I and implications for clinical trials. Neurology, 83(9), 810–817.
Talbot, K., & Tizzano, E. F. (2017). The clinical landscape for SMA in a new therapeutic era. Gene Therapy, 24(9), 529–533.
Brzustowicz, L. M., Lehner, T., Castilla, L. H., Penchaszadeh, G. K., Wilhelmsen, K. C., Daniels, R., Davies, K. E., Leppert, M., Ziter, F., Wood, D., et al. (1990). Genetic mapping of chronic childhood-onset spinal muscular atrophy to chromosome 5q11.2–13.3. Nature, 344(6266), 540–541.
Gilliam, T. C., Brzustowicz, L. M., Castilla, L. H., Lehner, T., Penchaszadeh, G. K., Daniels, R. J., Byth, B. C., Knowles, J., Hislop, J. E., Shapira, Y., et al. (1990). Genetic homogeneity between acute and chronic forms of spinal muscular atrophy. Nature, 345(6278), 823–825.
Melki, J., Abdelhak, S., Sheth, P., Bachelot, M. F., Burlet, P., Marcadet, A., Aicardi, J., Barois, A., Carriere, J. P., Fardeau, M., et al. (1990). Gene for chronic proximal spinal muscular atrophies maps to chromosome 5q. Nature, 344(6268), 767–768.
Munsat, T. L., Skerry, L., Korf, B., Pober, B., Schapira, Y., Gascon, G. G., al-Rajeh, S. M., Dubowitz, V., Davies, K., Brzustowicz, L. M., et al. (1990). Phenotypic heterogeneity of spinal muscular atrophy mapping to chromosome 5q11.2–13.3 (SMA 5q). Neurology, 40(12), 1831–1836.
Cobben, J. M., Scheffer, H., De Visser, M., Osinga, J., Frants, R., van der Steege, G., Wijmenga, C., ten Kate, L. P., van Ommen, G. J., & Buys, C. H. (1993). Linkage and apparent heterogeneity in proximal spinal muscular atrophies. Neuromuscular Disorders, 3(4), 327–333.
Brzustowicz, L. M., Kleyn, P. W., Boyce, F. M., Lien, L. L., Monaco, A. P., Penchaszadeh, G. K., Das, K., Wang, C. H., Munsat, T. L., Ott, J., et al. (1992). Fine-mapping of the spinal muscular atrophy locus to a region flanked by MAP1B and D5S6. Genomics, 13(4), 991–998.
Wirth, B., Pick, E., Leutner, A., Dadze, A., Voosen, B., Knapp, M., Piechaczek-Wappenschmidt, B., Rudnik-Schoneborn, S., Schonling, J., Cox, S., et al. (1994). Large linkage analysis in 100 families with autosomal recessive spinal muscular atrophy (SMA) and 11 CEPH families using 15 polymorphic loci in the region 5q11.2-q13.3. Genomics, 20(1), 84–93.
Bussaglia, E., Clermont, O., Tizzano, E., Lefebvre, S., Burglen, L., Cruaud, C., Urtizberea, J. A., Colomer, J., Munnich, A., Baiget, M., et al. (1995). A frame-shift deletion in the survival motor neuron gene in Spanish spinal muscular atrophy patients. Nature Genetics, 11(3), 335–337.
Clermont, O., Burlet, P., Lefebvre, S., Burglen, L., Munnich, A., & Melki, J. (1995). SMN gene deletions in adult-onset spinal muscular atrophy. Lancet, 346(8991–8992), 1712–1713.
Cobben, J. M., van der Steege, G., Grootscholten, P., de Visser, M., Scheffer, H., & Buys, C. H. (1995). Deletions of the survival motor neuron gene in unaffected siblings of patients with spinal muscular atrophy. American Journal of Human Genetics, 57(4), 805–808.
Lefebvre, S., Burglen, L., Reboullet, S., Clermont, O., Burlet, P., Viollet, L., Benichou, B., Cruaud, C., Millasseau, P., Zeviani, M., et al. (1995). Identification and characterization of a spinal muscular atrophy-determining gene. Cell, 80(1), 155–165.
McAndrew, P. E., Parsons, D. W., Simard, L. R., Rochette, C., Ray, P. N., Mendell, J. R., Prior, T. W., & Burghes, A. H. (1997). Identification of proximal spinal muscular atrophy carriers and patients by analysis of SMNT and SMNC gene copy number. American Journal of Human Genetics, 60(6), 1411–1422.
Schwartz, M., Sorensen, N., Hansen, F. J., Hertz, J. M., Norby, S., Tranebjaerg, L., & Skovby, F. (1997). Quantification, by solid-phase minisequencing, of the telomeric and centromeric copies of the survival motor neuron gene in families with spinal muscular atrophy. Human Molecular Genetics, 6(1), 99–104.
Lefebvre, S., Burlet, P., Liu, Q., Bertrandy, S., Clermont, O., Munnich, A., Dreyfuss, G., & Melki, J. (1997). Correlation between severity and SMN protein level in spinal muscular atrophy. Nature Genetics, 16(3), 265–269.
Calucho, M., Bernal, S., Alias, L., March, F., Vencesla, A., Rodriguez-Alvarez, F. J., Aller, E., Fernandez, R. M., Borrego, S., Millan, J. M., Hernandez-Chico, C., Cusco, I., Fuentes-Prior, P., & Tizzano, E. F. (2018). Correlation between SMA type and SMN2 copy number revisited: An analysis of 625 unrelated Spanish patients and a compilation of 2834 reported cases. Neuromuscular Disorders, 28(3), 208–215.
Monani, U. R., Lorson, C. L., Parsons, D. W., Prior, T. W., Androphy, E. J., Burghes, A. H., & McPherson, J. D. (1999). A single nucleotide difference that alters splicing patterns distinguishes the SMA gene SMN1 from the copy gene SMN2. Human Molecular Genetics, 8(7), 1177–1183.
Murray, L. M., Talbot, K., & Gillingwater, T. H. (2010). Review: neuromuscular synaptic vulnerability in motor neurone disease: amyotrophic lateral sclerosis and spinal muscular atrophy. Neuropathology and Applied Neurobiology, 36(2), 133–156.
Lorson, C. L., & Androphy, E. J. (2000). An exonic enhancer is required for inclusion of an essential exon in the SMA-determining gene SMN. Human Molecular Genetics, 9(2), 259–265.
Germain-Desprez, D., Brun, T., Rochette, C., Semionov, A., Rouget, R., & Simard, L. R. (2001). The SMN genes are subject to transcriptional regulation during cellular differentiation. Gene, 279(2), 109–117.
La Bella, V., Cisterni, C., Salaun, D., & Pettmann, B. (1998). Survival motor neuron (SMN) protein in rat is expressed as different molecular forms and is developmentally regulated. The European Journal of Neuroscience, 10(9), 2913–2923.
La Bella, V., Kallenbach, S., & Pettmann, B. (2000). Expression and subcellular localization of two isoforms of the survival motor neuron protein in different cell types. Journal of Neuroscience Research, 62(3), 346–356.
Burlet, P., Huber, C., Bertrandy, S., Ludosky, M. A., Zwaenepoel, I., Clermont, O., Roume, J., Delezoide, A. L., Cartaud, J., Munnich, A., & Lefebvre, S. (1998). The distribution of SMN protein complex in human fetal tissues and its alteration in spinal muscular atrophy. Human Molecular Genetics, 7(12), 1927–1933.
Gavrilov, D. K., Shi, X., Das, K., Gilliam, T. C., & Wang, C. H. (1998). Differential SMN2 expression associated with SMA severity. Nature Genetics, 20(3), 230–231.
Glascock, J., Lenz, M., Hobby, K., & Jarecki, J. (2017). Cure SMA and our patient community celebrate the first approved drug for SMA. Gene Therapy, 24(9), 498–500.
Paton, D. M. (2017). Nusinersen: antisense oligonucleotide to increase SMN protein production in spinal muscular atrophy. Drugs Today (Barc), 53(6), 327–337.
Singh, N. N., Howell, M. D., Androphy, E. J., & Singh, R. N. (2017). How the discovery of ISS-N1 led to the first medical therapy for spinal muscular atrophy. Gene Therapy, 24(9), 520–526.
Mercuri, E., Darras, B. T., Chiriboga, C. A., Day, J. W., Campbell, C., Connolly, A. M., Iannaccone, S. T., Kirschner, J., Kuntz, N. L., Saito, K., Shieh, P. B., Tulinius, M., Mazzone, E. S., Montes, J., Bishop, K. M., Yang, Q., Foster, R., Gheuens, S., Bennett, C. F., Farwell, W., Schneider, E., De Vivo, D. C., Finkel, R. S., & Group C S. (2018). Nusinersen versus Sham Control in Later-Onset Spinal Muscular Atrophy. The New England Journal of Medicine, 378(7), 625–635.
Wurster, C. D., Winter, B., Wollinsky, K., Ludolph, A. C., Uzelac, Z., Witzel, S., Schocke, M., Schneider, R., & Kocak, T. (2019). Intrathecal administration of nusinersen in adolescent and adult SMA type 2 and 3 patients. Journal of Neurology, 266(1), 183–194.
Mendell, J. R., Al-Zaidy, S., Shell, R., Arnold, W. D., Rodino-Klapac, L. R., Prior, T. W., Lowes, L., Alfano, L., Berry, K., Church, K., Kissel, J. T., Nagendran, S., L'Italien, J., Sproule, D. M., Wells, C., Cardenas, J. A., Heitzer, M. D., Kaspar, A., Corcoran, S., Braun, L., Likhite, S., Miranda, C., Meyer, K., Foust, K. D., Burghes, A. H. M., & Kaspar, B. K. (2017). Single-Dose Gene-Replacement Therapy for Spinal Muscular Atrophy. The New England Journal of Medicine, 377(18), 1713–1722.
Prior, T. W., Krainer, A. R., Hua, Y., Swoboda, K. J., Snyder, P. C., Bridgeman, S. J., Burghes, A. H., & Kissel, J. T. (2009). A positive modifier of spinal muscular atrophy in the SMN2 gene. American Journal of Human Genetics, 85(3), 408–413.
Novelli, G., Calza, L., Amicucci, P., Giardino, L., Pozza, M., Silani, V., Pizzuti, A., Gennarelli, M., Piombo, G., Capon, F., & Dallapiccola, B. (1997). Expression study of survival motor neuron gene in human fetal tissues. Biochemical and Molecular Medicine, 61(1), 102–106.
Schrank, B., Gotz, R., Gunnersen, J. M., Ure, J. M., Toyka, K. V., Smith, A. G., & Sendtner, M. (1997). Inactivation of the survival motor neuron gene, a candidate gene for human spinal muscular atrophy, leads to massive cell death in early mouse embryos. Proceedings of the National Academy of Sciences of the United States of America, 94(18), 9920–9925.
Lorson, C. L., & Androphy, E. J. (1998). The domain encoded by exon 2 of the survival motor neuron protein mediates nucleic acid binding. Human Molecular Genetics, 7(8), 1269–1275.
Bertrandy, S., Burlet, P., Clermont, O., Huber, C., Fondrat, C., Thierry-Mieg, D., Munnich, A., & Lefebvre, S. (1999). The RNA-binding properties of SMN: deletion analysis of the zebrafish orthologue defines domains conserved in evolution. Human Molecular Genetics, 8(5), 775–782.
Young, P. J., Man, N. T., Lorson, C. L., Le, T. T., Androphy, E. J., Burghes, A. H., & Morris, G. E. (2000). The exon 2b region of the spinal muscular atrophy protein, SMN, is involved in self-association and SIP1 binding. Human Molecular Genetics, 9(19), 2869–2877.
Young, P. J., Day, P. M., Zhou, J., Androphy, E. J., Morris, G. E., & Lorson, C. L. (2002). A direct interaction between the survival motor neuron protein and p53 and its relationship to spinal muscular atrophy. The Journal of Biological Chemistry, 277(4), 2852–2859.
Parsons, D. W., McAndrew, P. E., Monani, U. R., Mendell, J. R., Burghes, A. H., & Prior, T. W. (1996). An 11 base pair duplication in exon 6 of the SMN gene produces a type I spinal muscular atrophy (SMA) phenotype: further evidence for SMN as the primary SMA-determining gene. Human Molecular Genetics, 5(11), 1727–1732.
Xu, C. C., Denton, K. R., Wang, Z. B., Zhang, X., & Li, X. J. (2016). Abnormal mitochondrial transport and morphology as early pathological changes in human models of spinal muscular atrophy. Disease Models & Mechanisms, 9(1), 39–49.
Bowerman, M., Becker, C. G., Yanez-Munoz, R. J., Ning, K., Wood, M. J. A., Gillingwater, T. H., Talbot, K., & Consortium, U. S. R. (2017). Therapeutic strategies for spinal muscular atrophy: SMN and beyond. Disease Models & Mechanisms, 10(8), 943–954.
Chari, A., Paknia, E., & Fischer, U. (2009). The role of RNP biogenesis in spinal muscular atrophy. Current Opinion in Cell Biology, 21(3), 387–393.
Neuenkirchen, N., Englbrecht, C., Ohmer, J., Ziegenhals, T., Chari, A., & Fischer, U. (2015). Reconstitution of the human U snRNP assembly machinery reveals stepwise Sm protein organization. The EMBO Journal, 34(14), 1925–1941.
Burghes, A. H., & Beattie, C. E. (2009). Spinal muscular atrophy: why do low levels of survival motor neuron protein make motor neurons sick? Nature Reviews. Neuroscience, 10(8), 597–609.
Meister, G., Buhler, D., Laggerbauer, B., Zobawa, M., Lottspeich, F., & Fischer, U. (2000). Characterization of a nuclear 20S complex containing the survival of motor neurons (SMN) protein and a specific subset of spliceosomal Sm proteins. Human Molecular Genetics, 9(13), 1977–1986.
Meister, G., Eggert, C., & Fischer, U. (2002). SMN-mediated assembly of RNPs: a complex story. Trends in Cell Biology, 12(10), 472–478.
Malatesta, M., Scassellati, C., Meister, G., Plottner, O., Buhler, D., Sowa, G., Martin, T. E., Keidel, E., Fischer, U., & Fakan, S. (2004). Ultrastructural characterisation of a nuclear domain highly enriched in survival of motor neuron (SMN) protein. Experimental Cell Research, 292(2), 312–321.
Patel, A. A., McCarthy, M., & Steitz, J. A. (2002). The splicing of U12-type introns can be a rate-limiting step in gene expression. The EMBO Journal, 21(14), 3804–3815.
Boulisfane, N., Choleza, M., Rage, F., Neel, H., Soret, J., & Bordonne, R. (2011). Impaired minor tri-snRNP assembly generates differential splicing defects of U12-type introns in lymphoblasts derived from a type I SMA patient. Human Molecular Genetics, 20(4), 641–648.
Rage, F., Boulisfane, N., Rihan, K., Neel, H., Gostan, T., Bertrand, E., Bordonne, R., & Soret, J. (2013). Genome-wide identification of mRNAs associated with the protein SMN whose depletion decreases their axonal localization. RNA, 19(12), 1755–1766.
Lotti, F., Imlach, W. L., Saieva, L., Beck, E. S., Hao le, T., Li, D. K., Jiao, W., Mentis, G. Z., Beattie, C. E., McCabe, B. D., & Pellizzoni, L. (2012). An SMN-Dependent U12 Splicing Event Essential for Motor Circuit Function. Cell, 151(2), 440–454.
Hosseinibarkooie, S., Schneider, S., & Wirth, B. (2017). Advances in understanding the role of disease-associated proteins in spinal muscular atrophy. Expert Review of Proteomics, 14(7), 581–592.
Burge, C. B., Padgett, R. A., & Sharp, P. A. (1998). Evolutionary fates and origins of U12-type introns. Molecular Cell, 2(6), 773–785.
Levine, A., & Durbin, R. (2001). A computational scan for U12-dependent introns in the human genome sequence. Nucleic Acids Research, 29(19), 4006–4013.
Rochette, C. F., Gilbert, N., & Simard, L. R. (2001). SMN gene duplication and the emergence of the SMN2 gene occurred in distinct hominids: SMN2 is unique to Homo sapiens. Human Genetics, 108(3), 255–266.
Liu, L. (2017). Linking Telomere Regulation to Stem Cell Pluripotency. Trends in Genetics, 33(1), 16–33.
De Coppi, P., Bartsch, G., Jr., Siddiqui, M. M., Xu, T., Santos, C. C., Perin, L., Mostoslavsky, G., Serre, A. C., Snyder, E. Y., Yoo, J. J., Furth, M. E., Soker, S., & Atala, A. (2007). Isolation of amniotic stem cell lines with potential for therapy. Nature Biotechnology, 25(1), 100–106.
Bottai, D., Cigognini, D., Nicora, E., Moro, M., Grimoldi, M. G., Adami, R., Abrignani, S., Marconi, A. M., Di Giulio, A. M., & Gorio, A. (2012). Third trimester amniotic fluid cells with the capacity to develop neural phenotypes and with heterogeneity among sub-populations. Restorative Neurology and Neuroscience, 30(1), 55–68.
Bottai, D., Scesa, G., Cigognini, D., Adami, R., Nicora, E., Abrignani, S., Di Giulio, A. M., & Gorio, A. (2014). Third trimester NG2-positive amniotic fluid cells are effective in improving repair in spinal cord injury. Experimental Neurology, 254, 121–133.
Cooper, R. N., Butler-Browne, G. S., & Mouly, V. (2006). Human muscle stem cells. Current Opinion in Pharmacology, 6(3), 295–300.
Reynolds, B. A., & Weiss, S. (1992). Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science, 255(5052), 1707–1710.
Vescovi, A. L., Parati, E. A., Gritti, A., Poulin, P., Ferrario, M., Wanke, E., Frolichsthal-Schoeller, P., Cova, L., Arcellana-Panlilio, M., Colombo, A., & Galli, R. (1999). Isolation and cloning of multipotential stem cells from the embryonic human CNS and establishment of transplantable human neural stem cell lines by epigenetic stimulation. Experimental Neurology, 156(1), 71–83.
Gage, F. H. (2000). Mammalian neural stem cells. Science, 287(5457), 1433–1438.
Daniela, F., Vescovi, A. L., & Bottai, D. (2007). The stem cells as a potential treatment for neurodegeneration. Methods in Molecular Biology, 399, 199–213.
Adami, R., Scesa, G., & Bottai, D. (2014). Stem cell transplantation in neurological diseases: improving effectiveness in animal models. Frontiers in Cell and Development Biology, 2, 17.
Adami, R., Pagano, J., Colombo, M., Platonova, N., Recchia, D., Chiaramonte, R., Bottinelli, R., Canepari, M., & Bottai, D. (2018). Reduction of Movement in Neurological Diseases: Effects on Neural Stem Cells Characteristics. Frontiers in Neuroscience, 12, 336.
Bottai, D., Adami, R., & Ghidoni, R. (2018). Glycosphingolipids and Neural Stem Cells. Journal of Neurochemistry, 148(6), 698–711.
Bottai, D., Spreafico, M., Pistocchi, A., Fazio, G., Adami, R., Grazioli, P., Canu, A., Bragato, C., Rigamonti, S., Parodi, C., Cazzaniga, G., Biondi, A., Cotelli, F., Selicorni, A., & Massa, V. (2018). Modeling Cornelia de Lange Syndrome in vitro and in vivo reveals a role for cohesin complex in neuronal survival and differentiation. Human Molecular Genetics, 28(1), 64–73.
Maherali, N., Sridharan, R., Xie, W., Utikal, J., Eminli, S., Arnold, K., Stadtfeld, M., Yachechko, R., Tchieu, J., Jaenisch, R., Plath, K., & Hochedlinger, K. (2007). Directly reprogrammed fibroblasts show global epigenetic remodeling and widespread tissue contribution. Cell Stem Cell, 1(1), 55–70.
Takahashi, K., Tanabe, K., Ohnuki, M., Narita, M., Ichisaka, T., Tomoda, K., & Yamanaka, S. (2007). Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell, 131(5), 861–872.
Wernig, M., Meissner, A., Foreman, R., Brambrink, T., Ku, M., Hochedlinger, K., Bernstein, B. E., & Jaenisch, R. (2007). In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature, 448(7151), 318–324.
Yu, J., Vodyanik, M. A., Smuga-Otto, K., Antosiewicz-Bourget, J., Frane, J. L., Tian, S., Nie, J., Jonsdottir, G. A., Ruotti, V., Stewart, R., Slukvin, I. I., & Thomson, J. A. (2007). Induced pluripotent stem cell lines derived from human somatic cells. Science, 318(5858), 1917–1920.
Okita, K., Nakagawa, M., Hyenjong, H., Ichisaka, T., & Yamanaka, S. (2008). Generation of mouse induced pluripotent stem cells without viral vectors. Science, 322(5903), 949–953.
Park, I. H., Lerou, P. H., Zhao, R., Huo, H., & Daley, G. Q. (2008). Generation of human-induced pluripotent stem cells. Nature Protocols, 3(7), 1180–1186.
Park, I. H., Zhao, R., West, J. A., Yabuuchi, A., Huo, H., Ince, T. A., Lerou, P. H., Lensch, M. W., & Daley, G. Q. (2008). Reprogramming of human somatic cells to pluripotency with defined factors. Nature, 451(7175), 141–146.
Stadtfeld, M., Nagaya, M., Utikal, J., Weir, G., & Hochedlinger, K. (2008). Induced pluripotent stem cells generated without viral integration. Science, 322(5903), 945–949.
Okita, K., Matsumura, Y., Sato, Y., Okada, A., Morizane, A., Okamoto, S., Hong, H., Nakagawa, M., Tanabe, K., Tezuka, K., Shibata, T., Kunisada, T., Takahashi, M., Takahashi, J., Saji, H., & Yamanaka, S. (2011). A more efficient method to generate integration-free human iPS cells. Nature Methods, 8(5), 409–412.
Yoshioka, N., Gros, E., Li, H. R., Kumar, S., Deacon, D. C., Maron, C., Muotri, A. R., Chi, N. C., Fu, X. D., Yu, B. D., & Dowdy, S. F. (2013). Efficient generation of human iPSCs by a synthetic self-replicative RNA. Cell Stem Cell, 13(2), 246–254.
Carey, B. W., Markoulaki, S., Hanna, J., Saha, K., Gao, Q., Mitalipova, M., & Jaenisch, R. (2009). Reprogramming of murine and human somatic cells using a single polycistronic vector. Proceedings of the National Academy of Sciences of the United States of America, 106(1), 157–162.
Chang, C. W., Lai, Y. S., Pawlik, K. M., Liu, K., Sun, C. W., Li, C., Schoeb, T. R., & Townes, T. M. (2009). Polycistronic lentiviral vector for "hit and run" reprogramming of adult skin fibroblasts to induced pluripotent stem cells. Stem Cells, 27(5), 1042–1049.
Anokye-Danso, F., Trivedi, C. M., Juhr, D., Gupta, M., Cui, Z., Tian, Y., Zhang, Y., Yang, W., Gruber, P. J., Epstein, J. A., & Morrisey, E. E. (2011). Highly efficient miRNA-mediated reprogramming of mouse and human somatic cells to pluripotency. Cell Stem Cell, 8(4), 376–388.
Seo, B. J., Hong, Y. J., & Do, J. T. (2017). Cellular reprogramming using protein and cell-penetrating peptides. International Journal of Molecular Sciences, 18(3). https://doi.org/10.3390/ijms18030552.
Pappas, J. J., & Yang, P. C. (2008). Human ESC vs. iPSC-pros and cons. Journal of Cardiovascular Translational Research, 1(2), 96–99.
Li, Y., Balasubramanian, U., Cohen, D., Zhang, P. W., Mosmiller, E., Sattler, R., Maragakis, N. J., & Rothstein, J. D. (2015). A comprehensive library of familial human amyotrophic lateral sclerosis induced pluripotent stem cells. PLoS One, 10(3), e0118266.
Jaiswal, M. K. (2017). Therapeutic opportunities and challenges of induced pluripotent stem cells-derived motor neurons for treatment of amyotrophic lateral sclerosis and motor neuron disease. Neural Regeneration Research, 12(5), 723–736.
Madill, M., McDonagh, K., Ma, J., Vajda, A., McLoughlin, P., O'Brien, T., Hardiman, O., & Shen, S. (2017). Amyotrophic lateral sclerosis patient iPSC-derived astrocytes impair autophagy via non-cell autonomous mechanisms. Molecular Brain, 10(1), 22.
Terzic, D., Maxon, J. R., Krevitt, L., DiBartolomeo, C., Goyal, T., Low, W. C., Dutton, J. R., & Parr, A. M. (2016). Directed Differentiation of Oligodendrocyte Progenitor Cells From Mouse Induced Pluripotent Stem Cells. Cell Transplantation, 25(2), 411–424.
Xie, C., Liu, Y. Q., Guan, Y. T., & Zhang, G. X. (2016). Induced Stem Cells as a Novel Multiple Sclerosis Therapy. Current Stem Cell Research & Therapy, 11(4), 313–320.
Nicaise, A. M., Banda, E., Guzzo, R. M., Russomanno, K., Castro-Borrero, W., Willis, C. M., Johnson, K. M., Lo, A. C., & Crocker, S. J. (2017). iPS-derived neural progenitor cells from PPMS patients reveal defect in myelin injury response. Experimental Neurology, 288, 114–121.
Rinaldi, F., & Caldwell, M. A. (2013). Modeling astrocytic contribution toward neurodegeneration with pluripotent stem cells: focus on Alzheimer's and Parkinson's diseases. Neuroreport, 24(18), 1053–1057.
Majolo, F., Marinowic, D. R., Machado, D. C., & Da Costa, J. C. (2019). Important advances in Alzheimer's disease from the use of induced pluripotent stem cells. Journal of Biomedical Science, 26(1), 15.
Peteri, U. K., Niukkanen, M., & Castren, M. L. (2019). Astrocytes in Neuropathologies Affecting the Frontal Cortex. Frontiers in Cellular Neuroscience, 13, 44.
Daadi, M. M. (2019). Differentiation of Neural Stem Cells Derived from Induced Pluripotent Stem Cells into Dopaminergic Neurons. Methods in Molecular Biology, 1919, 89–96.
Stoddard-Bennett, T., & Reijo Pera, R. (2019). Treatment of Parkinson's Disease through Personalized Medicine and Induced Pluripotent Stem Cells. Cells, 8(1). https://doi.org/10.3390/cells8010026.
Abad, M., Mosteiro, L., Pantoja, C., Canamero, M., Rayon, T., Ors, I., Grana, O., Megias, D., Dominguez, O., Martinez, D., Manzanares, M., Ortega, S., & Serrano, M. (2013). Reprogramming in vivo produces teratomas and iPS cells with totipotency features. Nature, 502(7471), 340–345.
Chin, M. H., Mason, M. J., Xie, W., Volinia, S., Singer, M., Peterson, C., Ambartsumyan, G., Aimiuwu, O., Richter, L., Zhang, J., Khvorostov, I., Ott, V., Grunstein, M., Lavon, N., Benvenisty, N., Croce, C. M., Clark, A. T., Baxter, T., Pyle, A. D., Teitell, M. A., Pelegrini, M., Plath, K., & Lowry, W. E. (2009). Induced pluripotent stem cells and embryonic stem cells are distinguished by gene expression signatures. Cell Stem Cell, 5(1), 111–123.
Maherali, N., Ahfeldt, T., Rigamonti, A., Utikal, J., Cowan, C., & Hochedlinger, K. (2008). A high-efficiency system for the generation and study of human induced pluripotent stem cells. Cell Stem Cell, 3(3), 340–345.
Aoi, T., Yae, K., Nakagawa, M., Ichisaka, T., Okita, K., Takahashi, K., Chiba, T., & Yamanaka, S. (2008). Generation of pluripotent stem cells from adult mouse liver and stomach cells. Science, 321(5889), 699–702.
Miura, K., Okada, Y., Aoi, T., Okada, A., Takahashi, K., Okita, K., Nakagawa, M., Koyanagi, M., Tanabe, K., Ohnuki, M., Ogawa, D., Ikeda, E., Okano, H., & Yamanaka, S. (2009). Variation in the safety of induced pluripotent stem cell lines. Nature Biotechnology, 27(8), 743–745.
Charalambous, M., da Rocha, S. T., & Ferguson-Smith, A. C. (2007). Genomic imprinting, growth control and the allocation of nutritional resources: consequences for postnatal life. Current Opinion in Endocrinology, Diabetes, and Obesity, 14(1), 3–12.
Wilkinson, L. S., Davies, W., & Isles, A. R. (2007). Genomic imprinting effects on brain development and function. Nature Reviews. Neuroscience, 8(11), 832–843.
da Rocha, S. T., Edwards, C. A., Ito, M., Ogata, T., & Ferguson-Smith, A. C. (2008). Genomic imprinting at the mammalian Dlk1-Dio3 domain. Trends in Genetics, 24(6), 306–316.
Stadtfeld, M., Apostolou, E., Akutsu, H., Fukuda, A., Follett, P., Natesan, S., Kono, T., Shioda, T., & Hochedlinger, K. (2010). Aberrant silencing of imprinted genes on chromosome 12qF1 in mouse induced pluripotent stem cells. Nature, 465(7295), 175–181.
Ebert, A. D., Yu, J., Rose, F. F., Jr., Mattis, V. B., Lorson, C. L., Thomson, J. A., & Svendsen, C. N. (2009). Induced pluripotent stem cells from a spinal muscular atrophy patient. Nature, 457(7227), 277–280.
Garbes, L., Heesen, L., Holker, I., Bauer, T., Schreml, J., Zimmermann, K., Thoenes, M., Walter, M., Dimos, J., Peitz, M., Brustle, O., Heller, R., & Wirth, B. (2013). VPA response in SMA is suppressed by the fatty acid translocase CD36. Human Molecular Genetics, 22(2), 398–407.
Vazquez-Arango, P., Vowles, J., Browne, C., Hartfield, E., Fernandes, H. J., Mandefro, B., Sareen, D., James, W., Wade-Martins, R., Cowley, S. A., Murphy, S., & O'Reilly, D. (2016). Variant U1 snRNAs are implicated in human pluripotent stem cell maintenance and neuromuscular disease. Nucleic Acids Research, 44(22), 10960–10973.
Luo, Y., Fan, Y., Chen, X., Yu, B., Yue, L., Wang, D., Li, Q., Chen, Y., & Sun, X. (2012). Generation of Induced Pluripotent Stem Cells from Asian Patients with Chronic Neurodegenerative Diseases. The Journal of Reproduction and Development, 58(5), 515–521.
Spitalieri, P., Talarico, R. V., Botta, A., Murdocca, M., D'Apice, M. R., Orlandi, A., Giardina, E., Santoro, M., Brancati, F., Novelli, G., & Sangiuolo, F. (2015). Generation of Human Induced Pluripotent Stem Cells from Extraembryonic Tissues of Fetuses Affected by Monogenic Diseases. Cellular Reprogramming, 17(4), 275–287.
Murdocca, M., Ciafre, S. A., Spitalieri, P., Talarico, R. V., Sanchez, M., Novelli, G., & Sangiuolo, F. (2016). SMA Human iPSC-Derived Motor Neurons Show Perturbed Differentiation and Reduced miR-335-5p Expression. International Journal of Molecular Sciences, 17(8). https://doi.org/10.3390/ijms17081231.
Lin, X., Li, J. J., Qian, W. J., Zhang, Q. J., Wang, Z. F., Lu, Y. Q., Dong, E. L., He, J., Wang, N., Ma, L. X., & Chen, W. J. (2017). Modeling the differential phenotypes of spinal muscular atrophy with high-yield generation of motor neurons from human induced pluripotent stem cells. Oncotarget, 8(26), 42030–42042.
Feng, M., Liu, C., Xia, Y., Liu, B., Zhou, M., Li, Z., Sun, Q., Hu, Z., Wang, Y., Wu, L., Liu, X., & Liang, D. (2018). Restoration of SMN expression in mesenchymal stem cells derived from gene-targeted patient-specific iPSCs. Journal of Molecular Histology, 49(1), 27–37.
Hosoyama, T., McGivern, J. V., Van Dyke, J. M., Ebert, A. D., & Suzuki, M. (2014). Derivation of myogenic progenitors directly from human pluripotent stem cells using a sphere-based culture. Stem Cells Translational Medicine, 3(5), 564–574.
Hor, J. H., Soh, E. S., Tan, L. Y., Lim, V. J. W., Santosa, M. M., Winanto, Ho, B. X., Fan, Y., Soh, B. S., Ng, S. Y. (2018). Cell cycle inhibitors protect motor neurons in an organoid model of Spinal Muscular Atrophy Cell Death & Disease, 9(11), 1100. https://doi.org/10.1038/s41419-018-1081-0.
Chang, T., Zheng, W., Tsark, W., Bates, S., Huang, H., Lin, R. J., & Yee, J. K. (2011). Brief report: phenotypic rescue of induced pluripotent stem cell-derived motoneurons of a spinal muscular atrophy patient. Stem Cells, 29(12), 2090–2093.
Sareen, D., Ebert, A. D., Heins, B. M., McGivern, J. V., Ornelas, L., & Svendsen, C. N. (2012). Inhibition of apoptosis blocks human motor neuron cell death in a stem cell model of spinal muscular atrophy. PLoS One, 7(6), e39113.
Corti, S., Nizzardo, M., Simone, C., Falcone, M., Nardini, M., Ronchi, D., Donadoni, C., Salani, S., Riboldi, G., Magri, F., Menozzi, G., Bonaglia, C., Rizzo, F., Bresolin, N., & Comi, G. P. (2012). Genetic correction of human induced pluripotent stem cells from patients with spinal muscular atrophy. Science Translational Medicine, 4(165), 165ra162.
McGivern, J. V., Patitucci, T. N., Nord, J. A., Barabas, M. A., Stucky, C. L., & Ebert, A. D. (2013). Spinal muscular atrophy astrocytes exhibit abnormal calcium regulation and reduced growth factor production. Glia, 61(9), 1418–1428.
Schwab, A. J., & Ebert, A. D. (2014). Sensory neurons do not induce motor neuron loss in a human stem cell model of spinal muscular atrophy. PLoS One, 9(7), e103112.
Rindt, H., Feng, Z., Mazzasette, C., Glascock, J. J., Valdivia, D., Pyles, N., Crawford, T. O., Swoboda, K. J., Patitucci, T. N., Ebert, A. D., Sumner, C. J., Ko, C. P., & Lorson, C. L. (2015). Astrocytes influence the severity of spinal muscular atrophy. Human Molecular Genetics, 24(14), 4094–4102.
Yoshida, M., Kitaoka, S., Egawa, N., Yamane, M., Ikeda, R., Tsukita, K., Amano, N., Watanabe, A., Morimoto, M., Takahashi, J., Hosoi, H., Nakahata, T., Inoue, H., & Saito, M. K. (2015). Modeling the early phenotype at the neuromuscular junction of spinal muscular atrophy using patient-derived iPSCs. Stem Cell Reports, 4(4), 561–568.
Boza-Moran, M. G., Martinez-Hernandez, R., Bernal, S., Wanisch, K., Also-Rallo, E., Le Heron, A., Alias, L., Denis, C., Girard, M., Yee, J. K., Tizzano, E. F., & Yanez-Munoz, R. J. (2015). Decay in survival motor neuron and plastin 3 levels during differentiation of iPSC-derived human motor neurons. Scientific Reports, 5, 11696.
Liu, H., Lu, J., Chen, H., Du, Z., Li, X. J., & Zhang, S. C. (2015). Spinal muscular atrophy patient-derived motor neurons exhibit hyperexcitability. Scientific Reports, 5, 12189.
Ng, S. Y., Soh, B. S., Rodriguez-Muela, N., Hendrickson, D. G., Price, F., Rinn, J. L., & Rubin, L. L. (2015). Genome-wide RNA-Seq of Human Motor Neurons Implicates Selective ER Stress Activation in Spinal Muscular Atrophy. Cell Stem Cell, 17(5), 569–584.
Nizzardo, M., Simone, C., Dametti, S., Salani, S., Ulzi, G., Pagliarani, S., Rizzo, F., Frattini, E., Pagani, F., Bresolin, N., Comi, G., & Corti, S. (2015). Spinal muscular atrophy phenotype is ameliorated in human motor neurons by SMN increase via different novel RNA therapeutic approaches. Scientific Reports, 5, 11746.
Fuller, H. R., Mandefro, B., Shirran, S. L., Gross, A. R., Kaus, A. S., Botting, C. H., Morris, G. E., & Sareen, D. (2016). Spinal Muscular Atrophy Patient iPSC-Derived Motor Neurons Have Reduced Expression of Proteins Important in Neuronal Development. Frontiers in Cellular Neuroscience, 9, 506.
Patitucci, T. N., & Ebert, A. D. (2016). SMN deficiency does not induce oxidative stress in SMA iPSC-derived astrocytes or motor neurons. Human Molecular Genetics, 25(3), 514–523.
Powis, R. A., Karyka, E., Boyd, P., Come, J., Jones, R. A., Zheng, Y., Szunyogova, E., Groen, E. J., Hunter, G., Thomson, D., Wishart, T. M., Becker, C. G., Parson, S. H., Martinat, C., Azzouz, M., & Gillingwater, T. H. (2016). Systemic restoration of UBA1 ameliorates disease in spinal muscular atrophy. JCI Insight, 1(11), e87908.
Heesen, L., Peitz, M., Torres-Benito, L., Holker, I., Hupperich, K., Dobrindt, K., Jungverdorben, J., Ritzenhofen, S., Weykopf, B., Eckert, D., Hosseini-Barkooie, S. M., Storbeck, M., Fusaki, N., Lonigro, R., Heller, R., Kye, M. J., Brustle, O., & Wirth, B. (2016). Plastin 3 is upregulated in iPSC-derived motoneurons from asymptomatic SMN1-deleted individuals. Cellular and Molecular Life Sciences, 73(10), 2089–2104.
Osman, E. Y., Washington, C. W., 3rd, Kaifer, K. A., Mazzasette, C., Patitucci, T. N., Florea, K. M., Simon, M. E., Ko, C. P., Ebert, A. D., & Lorson, C. L. (2016). Optimization of Morpholino Antisense Oligonucleotides Targeting the Intronic Repressor Element1 in Spinal Muscular Atrophy. Molecular Therapy, 24(9), 1592–1601.
Ohuchi, K., Funato, M., Kato, Z., Seki, J., Kawase, C., Tamai, Y., Ono, Y., Nagahara, Y., Noda, Y., Kameyama, T., Ando, S., Tsuruma, K., Shimazawa, M., Hara, H., & Kaneko, H. (2016). Established Stem Cell Model of Spinal Muscular Atrophy Is Applicable in the Evaluation of the Efficacy of Thyrotropin-Releasing Hormone Analog. Stem Cells Translational Medicine, 5(2), 152–163.
Martin, J. E., Nguyen, T. T., Grunseich, C., Nofziger, J. H., Lee, P. R., Fields, D., Fischbeck, K. H., & Foran, E. (2017). Decreased Motor Neuron Support by SMA Astrocytes due to Diminished MCP1 Secretion. The Journal of Neuroscience, 37(21), 5309–5318.
Ando, S., Funato, M., Ohuchi, K., Kameyama, T., Inagaki, S., Seki, J., Kawase, C., Tsuruma, K., Shimazawa, M., Kaneko, H., & Hara, H. (2017). Edaravone is a candidate agent for spinal muscular atrophy: In vitro analysis using a human induced pluripotent stem cells-derived disease model. European Journal of Pharmacology, 814, 161–168.
Woo, C. J., Maier, V. K., Davey, R., Brennan, J., Li, G., Brothers, J., 2nd, Schwartz, B., Gordo, S., Kasper, A., Okamoto, T. R., Johansson, H. E., Mandefro, B., Sareen, D., Bialek, P., Chau, B. N., Bhat, B., Bullough, D., & Barsoum, J. (2017). Gene activation of SMN by selective disruption of lncRNA-mediated recruitment of PRC2 for the treatment of spinal muscular atrophy. Proceedings of the National Academy of Sciences of the United States of America, 114(8), E1509–E1518.
Palomo, V., Perez, D. I., Roca, C., Anderson, C., Rodriguez-Muela, N., Perez, C., Morales-Garcia, J. A., Reyes, J. A., Campillo, N. E., Perez-Castillo, A. M., Rubin, L. L., Timchenko, L., Gil, C., & Martinez, A. (2017). Subtly Modulating Glycogen Synthase Kinase 3 beta: Allosteric Inhibitor Development and Their Potential for the Treatment of Chronic Diseases. Journal of Medicinal Chemistry, 60(12), 4983–5001.
Sison, S. L., Patitucci, T. N., Seminary, E. R., Villalon, E., Lorson, C. L., & Ebert, A. D. (2017). Astrocyte-produced miR-146a as a mediator of motor neuron loss in spinal muscular atrophy. Human Molecular Genetics, 26(17), 3409–3420.
Lai, J. I., Leman, L. J., Ku, S., Vickers, C. J., Olsen, C. A., Montero, A., Ghadiri, M. R., & Gottesfeld, J. M. (2017). Cyclic tetrapeptide HDAC inhibitors as potential therapeutics for spinal muscular atrophy: Screening with iPSC-derived neuronal cells. Bioorganic & Medicinal Chemistry Letters, 27(15), 3289–3293.
d'Ydewalle, C., Ramos, D. M., Pyles, N. J., Ng, S. Y., Gorz, M., Pilato, C. M., Ling, K., Kong, L., Ward, A. J., Rubin, L. L., Rigo, F., Bennett, C. F., & Sumner, C. J. (2017). The Antisense Transcript SMN-AS1 Regulates SMN Expression and Is a Novel Therapeutic Target for Spinal Muscular Atrophy. Neuron, 93(1), 66–79.
Zhou, M., Hu, Z., Qiu, L., Zhou, T., Feng, M., Hu, Q., Zeng, B., Li, Z., Sun, Q., Wu, Y., Liu, X., Wu, L., & Liang, D. (2018). Seamless Genetic Conversion of SMN2 to SMN1 via CRISPR/Cpf1 and Single-Stranded Oligodeoxynucleotides in Spinal Muscular Atrophy Patient-Specific Induced Pluripotent Stem Cells. Human Gene Therapy, 29(11), 1252–1263.
Ramirez, A., Crisafulli, S. G., Rizzuti, M., Bresolin, N., Comi, G. P., Corti, S., & Nizzardo, M. (2018). Investigation of New Morpholino Oligomers to Increase Survival Motor Neuron Protein Levels in Spinal Muscular Atrophy. International Journal of Molecular Sciences, 19(1). https://doi.org/10.3390/ijms19010167.
Ohuchi, K., Funato, M., Ando, S., Inagaki, S., Sato, A., Kawase, C., Seki, J., Nakamura, S., Shimazawa, M., Kaneko, H., & Hara, H. (2019). Impairment of oligodendrocyte lineages in spinal muscular atrophy model systems. Neuroreport, 30(5), 350–357.
Son, Y. S., Choi, K., Lee, H., Kwon, O., Jung, K. B., Cho, S., Baek, J., Son, B., Kang, S. M., Kang, M., Yoon, J., Shen, H., Lee, S., Oh, J. H., Lee, H. A., Lee, M. O., Cho, H. S., Jung, C. R., Kim, J., Cho, S., & Son, M. Y. (2019). A SMN2 Splicing Modifier Rescues the Disease Phenotypes in an In Vitro Human Spinal Muscular Atrophy Model. Stem Cells and Development, 28(7), 438–453.
Sareen, D., van Ginkel, P. R., Takach, J. C., Mohiuddin, A., Darjatmoko, S. R., Albert, D. M., & Polans, A. S. (2006). Mitochondria as the primary target of resveratrol-induced apoptosis in human retinoblastoma cells. Investigative Ophthalmology & Visual Science, 47(9), 3708–3716.
Kramer, N. J., & Gitler, A. D. (2017). Raise the Roof: Boosting the Efficacy of a Spinal Muscular Atrophy Therapy. Neuron, 93(1), 3–5.
Ackermann, B., Krober, S., Torres-Benito, L., Borgmann, A., Peters, M., Hosseini Barkooie, S. M., Tejero, R., Jakubik, M., Schreml, J., Milbradt, J., Wunderlich, T. F., Riessland, M., Tabares, L., & Wirth, B. (2013). Plastin 3 ameliorates spinal muscular atrophy via delayed axon pruning and improves neuromuscular junction functionality. Human Molecular Genetics, 22(7), 1328–1347.
Oprea, G. E., Krober, S., McWhorter, M. L., Rossoll, W., Muller, S., Krawczak, M., Bassell, G. J., Beattie, C. E., & Wirth, B. (2008). Plastin 3 is a protective modifier of autosomal recessive spinal muscular atrophy. Science, 320(5875), 524–527.
Bernal, S., Also-Rallo, E., Martinez-Hernandez, R., Alias, L., Rodriguez-Alvarez, F. J., Millan, J. M., Hernandez-Chico, C., Baiget, M., & Tizzano, E. F. (2011). Plastin 3 expression in discordant spinal muscular atrophy (SMA) siblings. Neuromuscular Disorders, 21(6), 413–419.
Gabanella, F., Butchbach, M. E., Saieva, L., Carissimi, C., Burghes, A. H., & Pellizzoni, L. (2007). Ribonucleoprotein assembly defects correlate with spinal muscular atrophy severity and preferentially affect a subset of spliceosomal snRNPs. PLoS One, 2(9), e921.
Bach, J. R. (2007). Medical considerations of long-term survival of Werdnig-Hoffmann disease. American Journal of Physical Medicine & Rehabilitation, 86(5), 349–355.
Moller, P., Moe, N., Saugstad, O. D., Skullerud, K., Velken, M., Berg, K., Nitter-Hauge, S., & Borresen, A. L. (1990). Spinal muscular atrophy type I combined with atrial septal defect in three sibs. Clinical Genetics, 38(2), 81–83.
Tein, I., Sloane, A. E., Donner, E. J., Lehotay, D. C., Millington, D. S., & Kelley, R. I. (1995). Fatty acid oxidation abnormalities in childhood-onset spinal muscular atrophy: primary or secondary defect(s)? Pediatric Neurology, 12(1), 21–30.
Shababi, M., Lorson, C. L., & Rudnik-Schoneborn, S. S. (2014). Spinal muscular atrophy: a motor neuron disorder or a multi-organ disease? Journal of Anatomy, 224(1), 15–28.
Park, G. H., Maeno-Hikichi, Y., Awano, T., Landmesser, L. T., & Monani, U. R. (2010). Reduced survival of motor neuron (SMN) protein in motor neuronal progenitors functions cell autonomously to cause spinal muscular atrophy in model mice expressing the human centromeric (SMN2) gene. The Journal of Neuroscience, 30(36), 12005–12019.
Tong, X., Ao, Y., Faas, G. C., Nwaobi, S. E., Xu, J., Haustein, M. D., Anderson, M. A., Mody, I., Olsen, M. L., Sofroniew, M. V., & Khakh, B. S. (2014). Astrocyte Kir4.1 ion channel deficits contribute to neuronal dysfunction in Huntington's disease model mice. Nature Neuroscience, 17(5), 694–703.
Wolf, G. (2003). Growth factors and the development of diabetic nephropathy. Current Diabetes Reports, 3(6), 485–490.
He, Y., Huang, C., Sun, X., Long, X. R., Lv, X. W., & Li, J. (2012). MicroRNA-146a modulates TGF-beta1-induced hepatic stellate cell proliferation by targeting SMAD4. Cellular Signalling, 24(10), 1923–1930.
Huang, C., Day, M. L., Poronnik, P., Pollock, C. A., & Chen, X. M. (2014). Inhibition of KCa3.1 suppresses TGF-beta1 induced MCP-1 expression in human proximal tubular cells through Smad3, p38 and ERK1/2 signaling pathways. The International Journal of Biochemistry & Cell Biology, 47, 1–10.
Martinez-Hernandez, R., Bernal, S., Alias, L., & Tizzano, E. F. (2014). Abnormalities in early markers of muscle involvement support a delay in myogenesis in spinal muscular atrophy. Journal of Neuropathology and Experimental Neurology, 73(6), 559–567.
Valsecchi, V., Boido, M., De Amicis, E., Piras, A., & Vercelli, A. (2015). Expression of Muscle-Specific MiRNA 206 in the Progression of Disease in a Murine SMA Model. PLoS One, 10(6), e0128560.
Ripolone, M., Ronchi, D., Violano, R., Vallejo, D., Fagiolari, G., Barca, E., Lucchini, V., Colombo, I., Villa, L., Berardinelli, A., Balottin, U., Morandi, L., Mora, M., Bordoni, A., Fortunato, F., Corti, S., Parisi, D., Toscano, A., Sciacco, M., DiMauro, S., Comi, G. P., & Moggio, M. (2015). Impaired Muscle Mitochondrial Biogenesis and Myogenesis in Spinal Muscular Atrophy. JAMA Neurology, 72(6), 666–675.
Fayzullina, S., & Martin, L. J. (2016). DNA Damage Response and DNA Repair in Skeletal Myocytes From a Mouse Model of Spinal Muscular Atrophy. Journal of Neuropathology and Experimental Neurology, 75(9), 889–902.
Riessland, M., Brichta, L., Hahnen, E., & Wirth, B. (2006). The benzamide M344, a novel histone deacetylase inhibitor, significantly increases SMN2 RNA/protein levels in spinal muscular atrophy cells. Human Genetics, 120(1), 101–110.
Avila, A. M., Burnett, B. G., Taye, A. A., Gabanella, F., Knight, M. A., Hartenstein, P., Cizman, Z., Di Prospero, N. A., Pellizzoni, L., Fischbeck, K. H., & Sumner, C. J. (2007). Trichostatin A increases SMN expression and survival in a mouse model of spinal muscular atrophy. The Journal of Clinical Investigation, 117(3), 659–671.
Mercuri, E., Bertini, E., Messina, S., Solari, A., D'Amico, A., Angelozzi, C., Battini, R., Berardinelli, A., Boffi, P., Bruno, C., Cini, C., Colitto, F., Kinali, M., Minetti, C., Mongini, T., Morandi, L., Neri, G., Orcesi, S., Pane, M., Pelliccioni, M., Pini, A., Tiziano, F. D., Villanova, M., Vita, G., & Brahe, C. (2007). Randomized, double-blind, placebo-controlled trial of phenylbutyrate in spinal muscular atrophy. Neurology, 68(1), 51–55.
Swoboda, K. J., Scott, C. B., Crawford, T. O., Simard, L. R., Reyna, S. P., Krosschell, K. J., Acsadi, G., Elsheik, B., Schroth, M. K., D'Anjou, G., LaSalle, B., Prior, T. W., Sorenson, S. L., Maczulski, J. A., Bromberg, M. B., Chan, G. M., & Kissel, J. T. (2010). SMA CARNI-VAL trial part I: double-blind, randomized, placebo-controlled trial of L-carnitine and valproic acid in spinal muscular atrophy. PLoS One, 5(8), e12140.
Evans, M. C., Cherry, J. J., & Androphy, E. J. (2011). Differential regulation of the SMN2 gene by individual HDAC proteins. Biochemical and Biophysical Research Communications, 414(1), 25–30.
Nizzardo, M., Simone, C., Salani, S., Ruepp, M. D., Rizzo, F., Ruggieri, M., Zanetta, C., Brajkovic, S., Moulton, H. M., Muehlemann, O., Bresolin, N., Comi, G. P., & Corti, S. (2014). Effect of combined systemic and local morpholino treatment on the spinal muscular atrophy Delta7 mouse model phenotype. Clinical Therapeutics, 36(3), 340–356 e345.
Acknowledgements
We would like to thank Damian Randle’s proofreading and editorial service for the academic world and for business in the United Kingdom and Europe (http://englishedituk.co.uk) for the complete revision of the manuscript.
Funding
The work was supported by a grant to Daniele Bottai from Asamsi ONLUS via Prosciutta, 23–48018 Faenza (RA), Italy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that this research was conducted in the absence of any commercial or financial relationship that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Adami, R., Bottai, D. Spinal Muscular Atrophy Modeling and Treatment Advances by Induced Pluripotent Stem Cells Studies. Stem Cell Rev and Rep 15, 795–813 (2019). https://doi.org/10.1007/s12015-019-09910-6
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12015-019-09910-6