Abstract
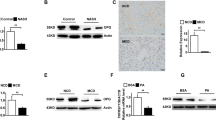
Neutrophils infiltration in liver is one of the typical histological characteristics of nonalcoholic steatohepatitis (NASH) in both animal models and human subjects. This study was aimed to investigate the role of neutrophils in the process of NASH and its underling mechanisms. C57BL/6J mice were fed with either standard chow (SC) or methionine/choline-deficient (MCD) diet for 1, 2, 4, 8 weeks, respectively. C57BL/6J APOE−/− mice were fed with SC or high-fat high-cholesterol (HFHC) diet. Anti-Ly6G antibody was employed to deplete neutrophils and sivelestat was used to inhibit neutrophil elastase (NE). MCD-diet-receiving mice with neutrophil depletion had much lower serum ALT activity, liver inflammation, and mRNA levels of proinflammatory genes in the early stage of NASH (1 and 2 weeks) when compared to non-neutrophil-depleted mice. NE inhibitor sivelestat could recapitulate the effects of neutrophil depletion in APOE−/− mice fed with HFHC diet. As the disease progressed (4 and 8 weeks), neutrophil depletion did not lower serum ALT levels and liver lesions due to activation of Kupffer cells. Finally, we found neutrophils also affected anti-inflammation cytokine interleukin-1 receptor antagonist mRNA expression. Neutrophils play a crucial role in the early stage of NASH via NE.




Similar content being viewed by others
References
Ong, J. P., Younossi, Z. M. (2007). Epidemiology and natural history of NAFLD and NASH. Clinics in Liver Disease, 11, 1–16, vii.
Xun, Y. H., Fan, J. G., Zang, G. Q., Liu, H., Jiang, Y. M., Xiang, J., et al. (2012). Suboptimal performance of simple noninvasive tests for advanced fibrosis in Chinese patients with nonalcoholic fatty liver disease. Journal of Digestive Diseases, 13, 588–595.
Rensen, S. S., Slaats, Y., Nijhuis, J., Jans, A., Bieghs, V., Driessen, A., et al. (2009). Increased hepatic myeloperoxidase activity in obese subjects with nonalcoholic steatohepatitis. American Journal of Pathology, 175, 1473–1482.
Gadd, V. L., Skoien, R., Powell, E. E., Fagan, K. J., Winterford, C., Horsfall, L., et al. (2014). The portal inflammatory infiltrate and ductular reaction in human nonalcoholic fatty liver disease. Hepatology, 59, 1393–1405.
Moles, A., Murphy, L., Wilson, C. L., Chakraborty, J. B., Fox, C., Park, E. J., et al. (2014). A TLR2/S100A9/CXCL-2 signaling network is necessary for neutrophil recruitment in acute and chronic liver injury in the mouse. Journal of Hepatology, 60, 782–791.
Alkhouri, N., Morris-Stiff, G., Campbell, C., Lopez, R., Tamimi, T. A., Yerian, L., et al. (2012). Neutrophil to lymphocyte ratio: a new marker for predicting steatohepatitis and fibrosis in patients with nonalcoholic fatty liver disease. Liver International, 32, 297–302.
Daley, J. M., Thomay, A. A., Connolly, M. D., Reichner, J. S., & Albina, J. E. (2008). Use of Ly6G-specific monoclonal antibody to deplete neutrophils in mice. Journal of Leukocyte Biology, 83, 64–70.
Pham, C. T. (2006). Neutrophil serine proteases: specific regulators of inflammation. Nature Reviews Immunology, 6, 541–550.
Talukdar, S., da Oh, Y., Bandyopadhyay, G., Li, D., Xu, J., McNelis, J., et al. (2012). Neutrophils mediate insulin resistance in mice fed a high-fat diet through secreted elastase. Nature Medicine, 18, 1407–1412.
Mansuy-Aubert, V., Zhou, Q. L., Xie, X., Gong, Z., Huang, J. Y., Khan, A. R., et al. (2013). Imbalance between neutrophil elastase and its inhibitor alpha1-antitrypsin in obesity alters insulin sensitivity, inflammation, and energy expenditure. Cell Metabolism, 17, 534–548.
Stankovic, M. N., Mladenovic, D. R., Duricic, I., Sobajic, S. S., Timic, J., Jorgacevic, B., et al. (2014). Time-dependent changes and association between liver free fatty acids, serum lipid profile and histological features in mice model of nonalcoholic fatty liver disease. Archives of Medical Research, 45, 116–124.
Ye, D., Li, F. Y., Lam, K. S., Li, H., Jia, W., Wang, Y., et al. (2012). Toll-like receptor-4 mediates obesity-induced non-alcoholic steatohepatitis through activation of X-box binding protein-1 in mice. Gut, 61, 1058–1067.
Kleiner, D. E., Brunt, E. M., Van Natta, M., Behling, C., Contos, M. J., Cummings, O. W., et al. (2005). Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology, 41, 1313–1321.
Liu, J., Zhuang, Z. J., Bian, D. X., Ma, X. J., Xun, Y. H., Yang, W. J., et al. (2014). Toll-like receptor-4 signalling in the progression of non-alcoholic fatty liver disease induced by high-fat and high-fructose diet in mice. Clinical and Experimental Pharmacology and Physiology, 41, 482–488.
Jamieson, T., Clarke, M., Steele, C. W., Samuel, M. S., Neumann, J., Jung, A., et al. (2012). Inhibition of CXCR2 profoundly suppresses inflammation-driven and spontaneous tumorigenesis. Journal of Clinical Investigation, 122, 3127–3144.
Zadelaar, S., Kleemann, R., Verschuren, L., de der Vries-Van Weij, J., van der Hoorn, J., Princen, H. M., et al. (2007). Mouse models for atherosclerosis and pharmaceutical modifiers. Arteriosclerosis, Thrombosis, and Vascular Biology, 27, 1706–1721.
Seki, E., Park, E., & Fujimoto, J. (2011). Toll-like receptor signaling in liver regeneration, fibrosis and carcinogenesis. Hepatology Research, 41, 597–610.
Seki, E., De Minicis, S., Osterreicher, C. H., Kluwe, J., Osawa, Y., Brenner, D. A., et al. (2007). TLR4 enhances TGF-beta signaling and hepatic fibrosis. Nature Medicine, 13, 1324–1332.
Freeman, B. D., & Buchman, T. G. (2001). Interleukin-1 receptor antagonist as therapy for inflammatory disorders. Expert Opinion on Biological Therapy, 1, 301–308.
Kavanaugh, A. (2006). Anakinra (interleukin-1 receptor antagonist) has positive effects on function and quality of life in patients with rheumatoid arthritis. Advances in Therapy, 23, 208–217.
Akash, M. S., Shen, Q., Rehman, K., & Chen, S. (2012). Interleukin-1 receptor antagonist: a new therapy for type 2 diabetes mellitus. Journal of Pharmaceutical Sciences, 101, 1647–1658.
Akash, M. S., Rehman, K., & Chen, S. (2013). An overview of valuable scientific models for diabetes mellitus. Current Diabetes Review, 9, 286–293.
Seckinger, P., Lowenthal, J. W., Williamson, K., Dayer, J. M., & MacDonald, H. R. (1987). A urine inhibitor of interleukin 1 activity that blocks ligand binding. Journal of Immunology, 139, 1546–1549.
Mocsai, A. (2013). Diverse novel functions of neutrophils in immunity, inflammation, and beyond. Journal of Experimental Medicine, 210, 1283–1299.
Drechsler, M., Megens, R. T., van Zandvoort, M., Weber, C., & Soehnlein, O. (2010). Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation, 122, 1837–1845.
Doring, Y., Drechsler, M., Wantha, S., Kemmerich, K., Lievens, D., Vijayan, S., et al. (2012). Lack of neutrophil-derived CRAMP reduces atherosclerosis in mice. Circulation Research, 110, 1052–1056.
von Bruhl, M. L., Stark, K., Steinhart, A., Chandraratne, S., Konrad, I., Lorenz, M., et al. (2012). Monocytes, neutrophils, and platelets cooperate to initiate and propagate venous thrombosis in mice in vivo. Journal of Experimental Medicine, 209, 819–835.
Piwowar, A., Knapik-Kordecka, M., & Warwas, M. (2000). Concentration of leukocyte elastase in plasma and polymorphonuclear neutrophil extracts in type 2 diabetes. Clinical Chemistry and Laboratory Medicine, 38, 1257–1261.
Bizbiz, L., Bonithon-Kopp, C., Ducimetiere, P., Berr, C., Alperovitch, A., & Robert, L. (1996). Relation of serum elastase activity to ultrasonographically assessed carotid artery wall lesions and cardiovascular risk factors. The EVA study. Atherosclerosis, 120, 47–55.
Takemasa, A., Ishii, Y., & Fukuda, T. (2012). A neutrophil elastase inhibitor prevents bleomycin-induced pulmonary fibrosis in mice. European Respiratory Journal, 40, 1475–1482.
Fox, J., & Haston, C. K. (2013). CXC receptor 1 and 2 and neutrophil elastase inhibitors alter radiation-induced lung disease in the mouse. International Journal of Radiation Oncology Biology Physics, 85, 215–222.
Sakashita, A., Nishimura, Y., Nishiuma, T., Takenaka, K., Kobayashi, K., Kotani, Y., et al. (2007). Neutrophil elastase inhibitor (sivelestat) attenuates subsequent ventilator-induced lung injury in mice. European Journal of Pharmacology, 571, 62–71.
Faria, M. S., Calegari-Silva, T. C., de Carvalho Vivarini, A., Mottram, J. C., Lopes, U. G., & Lima, A. P. (2014). Role of protein kinase R in the killing of Leishmania major by macrophages in response to neutrophil elastase and TLR4 via TNFalpha and IFNbeta. FASEB Journal, 28, 3050–3063.
McDonald, B., Jenne, C. N., Zhuo, L., Kimata, K., & Kubes, P. (2013). Kupffer cells and activation of endothelial TLR4 coordinate neutrophil adhesion within liver sinusoids during endotoxemia. American Journal of Physiology-Gastrointestinal and Liver Physiology, 305, G797–G806.
Serhan, C. N., Chiang, N., & Van Dyke, T. E. (2008). Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nature Reviews Immunology, 8, 349–361.
El Kebir, D., & Filep, J. G. (2010). Role of neutrophil apoptosis in the resolution of inflammation. Scientific World Journal, 10, 1731–1748.
Acknowledgments
This study was supported by Natural Science Foundation of Zhejiang Province (LY14H070004); Science and Technology Development Planning Project of Hangzhou City (20140633B09).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zang, S., Wang, L., Ma, X. et al. Neutrophils Play a Crucial Role in the Early Stage of Nonalcoholic Steatohepatitis via Neutrophil Elastase in Mice. Cell Biochem Biophys 73, 479–487 (2015). https://doi.org/10.1007/s12013-015-0682-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-015-0682-9