Abstract
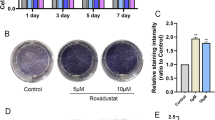
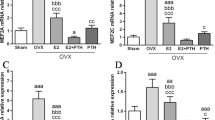
The hypoxia-inducible factor-1α (HIF-1α)/vascular endothelial growth factor (VEGF) pathway is involved in skeletal development, bone repair, and postmenopausal osteoporosis. Inhibitors of prolyl hydroxylases (PHD) enhance vascularity, increase callus formation in a stabilized fracture model, and activate the HIF-1α/VEGF pathway. This study examined the effects of estrogen on the HIF-1α/VEGF pathway in osteoblasts and whether PHD inhibitors can protect from bone loss in postmenopausal osteoporosis. Osteoblasts were treated with estrogen, and expressions of HIF-1α and VEGF were measured at mRNA (qPCR) and protein (Western blot) levels. Further, osteoblasts were treated with inhibitors of the phosphatidylinositol 3-kinase (PI3K)/protein kinase B (Akt) pathway, and levels of VEGF mRNA and protein expression were detected. In addition, ovariectomized rats were treated with PHD inhibitors, and bone microarchitecture and bone mechanical strength were assessed using micro-CT and biomechanical analyses (lower ultimate stress, modulus, and stiffness). Blood vessel formation was measured with India Ink Perfusion and immunohistochemistry. Estrogen, in a dose- and time-dependent manner, induced VEGF expression at both mRNA and protein levels and enhanced HIF-1α protein stability. Further, the estrogen-induced VEGF expression in osteoblasts involved the PI3K/Akt pathway. PHD inhibitors increased bone mineral density, bone microarchitecture and bone mechanical strength, and promoted blood vessel formation in ovariectomized rats. In conclusion, estrogen and PHD inhibitors activate the HIF-1α/VEGF pathway in osteoblasts. PHD inhibitors can be utilized to protect bone loss in postmenopausal osteoporosis by improving bone vascularity and angiogenesis in bone marrow.





Similar content being viewed by others
References
Harada, S., & Rodan, G. A. (2003). Control of osteoblast function and regulation of bone mass. Nature, 423, 349–355.
Boyle, W. J., Simonet, W. S., & Lacey, D. L. (2003). Osteoclast differentiation and activation. Nature, 423, 337–342.
Burkhardt, R., Kettner, G., Bohm, W., Schmidmeier, M., Schlag, R., Frisch, B., et al. (1987). Changes in trabecular bone, hematopoiesis and bone marrow vessels in aplastic anemia, primary osteoporosis, and old age: A comparative histomorphometric study. Bone, 8, 157–164.
Parfitt, A. M. (2000). The mechanism of coupling: A role for the vasculature. Bone, 2000(26), 319–323.
Vogt, M. T., Cauley, J. A., Kuller, L. H., & Nevitt, M. C. (1997). Bone mineral density and blood flow to the lower extremities: The study of osteoporotic fractures. Journal of Bone and Mineral Research, 12, 283–289.
Street, J. T., de Schrijver, K., Wang, J. H., Wu, Q. D., & Redmond, H. P. (2005). Hypoxia regulates the paracrine coupling of angiogenesis and bone formation. European Journal of Orthopaedic Surgery & Traumatology, 2005, 214–225.
Sanada, M., Taguchi, A., Higashi, Y., Tsuda, M., Kodama, I., Yoshizumi, M., et al. (2004). Forearm endothelial function and bone mineral loss in postmenopausal women. Atherosclerosis, 176, 387–392.
Semenza, G. L. (2003). Targeting HIF-1 for cancer therapy. Nature Reviews Cancer, 2003(3), 721–732.
Wang, Y., Wan, C., Deng, L., Liu, X., Cao, X., Gilbert, S. R., et al. (2007). The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. The Journal of Clinical Investigation, 117, 1616–1626.
Wan, C., Gilbert, S. R., Wang, Y., Cao, X., Shen, X., Ramaswamy, G., et al. (2008). Activation of the hypoxia-inducible factor-1alpha pathway accelerates bone regeneration. Proceedings to the National Academy of Science USA, 105, 686–691.
Zhao, Q., Shen, X., Zhang, W., Zhu, G., Qi, J., & Deng, L. (2012). Mice with increased angiogenesis and osteogenesis due to conditional activation of HIF pathway in osteoblasts are protected from ovariectomy induced bone loss. Bone, 50, 763–770.
Ivan, M., Kondo, K., Yang, H., Kim, W., Valiando, J., Ohh, M., et al. (2001). HIFalpha targeted for VHL-mediated destruction by proline hydroxylation: Implications for O2 sensing. Science, 292, 464–468.
Wang, G. L., & Semenza, G. L. (1993). Desferrioxamine induces erythropoietin gene expression and hypoxia-inducible factor 1 DNA-binding activity: Implications for models of hypoxia signal transduction. Blood, 82, 3610–3615.
Warnecke, C., Griethe, W., Weidemann, A., Jurgensen, J. S., Willam, C., Bachmann, S., et al. (2003). Activation of the hypoxia-inducible factor-pathway and stimulation of angiogenesis by application of prolyl hydroxylase inhibitors. FASEB Journal, 17, 1186–1188.
Chau, N. M., Rogers, P., Aherne, W., Carroll, V., Collins, I., McDonald, E., et al. (2005). Identification of novel small molecule inhibitors of hypoxia-inducible factor-1 that differentially block hypoxia-inducible factor-1 activity and hypoxia-inducible factor-1alpha induction in response to hypoxic stress and growth factors. Cancer Research, 65, 4918–4928.
Shen, X., Wan, C., Ramaswamy, G., Mavalli, M., Wang, Y., Duvall, C. L., et al. (2009). Prolyl hydroxylase inhibitors increase neoangiogenesis and callus formation following femur fracture in mice. Journal of Orthopaedic Research, 27, 1298–1305.
Castoria, G., Migliaccio, A., Bilancio, A., Di, D. M., de Falco, A., Lombardi, M., et al. (2001). PI3-kinase in concert with Src promotes the S-phase entry of oestradiol-stimulated MCF-7 cells. EMBO Journal, 20, 6050–6059.
Cabodi, S., Moro, L., Baj, G., Smeriglio, M., Di, S. P., Gippone, S., et al. (2004). p130Cas interacts with estrogen receptor alpha and modulates non-genomic estrogen signaling in breast cancer cells. Journal of Cell Science, 117, 1603–1611.
Honda, K., Sawada, H., Kihara, T., Urushitani, M., Nakamizo, T., Akaike, A., et al. (2000). Phosphatidylinositol 3-kinase mediates neuroprotection by estrogen in cultured cortical neurons. Journal of Neuroscience Research, 60, 321–327.
Yen, M. L., Su, J. L., Chien, C. L., Tseng, K. W., Yang, C. Y., Chen, W. F., et al. (2005). Diosgenin induces hypoxia-inducible factor-1 activation and angiogenesis through estrogen receptor-related phosphatidylinositol 3-kinase/Akt and p38 mitogen-activated protein kinase pathways in osteoblasts. Molecular Pharmacology, 68, 1061–1073.
Tivesten, A., Moverare-Skrtic, S., Chagin, A., Venken, K., Salmon, P., Vanderschueren, D., et al. (2004). Additive protective effects of estrogen and androgen treatment on trabecular bone in ovariectomized rats. Journal of Bone and Mineral Research, 19, 1833–1839.
Parfitt, A. M., Drezner, M. K., Glorieux, F. H., Kanis, J. A., Malluche, H., Meunier, P. J., et al. (1987). Bone histomorphometry: Standardization of nomenclature, symbols, and units. Report of the ASBMR Histomorphometry Nomenclature Committee. Journal of Bone and Mineral Research, 2, 595–610.
Shepherd, B. R., Chen, H. Y., Smith, C. M., Gruionu, G., Williams, S. K., & Hoying, J. B. (2004). Rapid perfusion and network remodeling in a microvascular construct after implantation. Arteriosclerosis, Thrombosis, and Vascular Biology, 24, 898–904.
Kennedy, J. M., & Zochodne, D. W. (2002). Influence of experimental diabetes on the microcirculation of injured peripheral nerve: Functional and morphological aspects. Diabetes, 51, 2233–2240.
Parfitt, A. M. (1994). Osteonal and hemi-osteonal remodeling: The spatial and temporal framework for signal traffic in adult human bone. Journal of Cellular Biochemistry, 1994(55), 273–286.
Parfitt, A. M. (1998). Osteoclast precursors as leukocytes: Importance of the area code. Bone, 1998(23), 491–494.
Ding, W. G., Wei, Z. X., & Liu, J. B. (2011). Reduced local blood supply to the tibial metaphysis is associated with ovariectomy-induced osteoporosis in mice. Connective Tissue Research, 52, 25–29.
Richelson, L. S., Wahner, H. W., Melton, L. J., I. I. I., & Riggs, B. L. (1984). Relative contributions of aging and estrogen deficiency to postmenopausal bone loss. New England Journal of Medicine, 311, 1273–1275.
Kang, D. H., Yu, E. S., Yoon, K. I., & Johnson, R. (2004). The impact of gender on progression of renal disease: Potential role of estrogen-mediated vascular endothelial growth factor regulation and vascular protection. American Journal of Pathology, 164, 679–688.
Sugino, N., Kashida, S., Takiguchi, S., Karube, A., & Kato, H. (2000). Expression of vascular endothelial growth factor and its receptors in the human corpus luteum during the menstrual cycle and in early pregnancy. Journal of Clinical Endocrinology and Metabolism, 85, 3919–3924.
Kazi, A. A., Jones, J. M., & Koos, R. D. (2005). Chromatin immunoprecipitation analysis of gene expression in the rat uterus in vivo: Estrogen-induced recruitment of both estrogen receptor alpha and hypoxia-inducible factor 1 to the vascular endothelial growth factor promoter. Molecular Endocrinology, 19, 2006–2019.
Kazi, A. A., & Koos, R. D. (2007). Estrogen-induced activation of hypoxia-inducible factor-1alpha, vascular endothelial growth factor expression, and edema in the uterus are mediated by the phosphatidylinositol 3-kinase/Akt pathway. Endocrinology, 2007, 2363–2374.
Pufe, T., Claasen, H., Scholz-Ahrens, K. E., Varoga, D., Drescher, W., Franke, A. T., et al. (2007). Influence of estradiol on vascular endothelial growth factor expression in bone: A study in Göttingen miniature pigs and human osteoblasts. Calcified Tissue International, 2007, 184–191.
Ivanova, T., Mendez, P., Garcia-Segura, L. M., & Beyer, C. (2002). Rapid stimulation of the PI3-kinase/Akt signalling pathway in developing midbrain neurones by oestrogen. Journal of Neuroendocrinology, 14, 73–79.
Sun, M., Paciga, J. E., Feldman, R. I., Yuan, Z., Coppola, D., Lu, Y. Y., et al. (2001). Phosphatidylinositol-3-OH kinase (PI3K)/AKT2, activated in breast cancer, regulates and is induced by estrogen receptor alpha (ERalpha) via interaction between ERalpha and PI3K. Cancer Research, 61, 5985–5991.
Lengyel, F., Vertes, Z., Kovacs, K. A., Kornyei, J. L., Sumegi, B., & Vertes, M. (2004). Expression and activation of Akt/protein kinase B in sexually immature and mature rat uterus. Journal of Steroid Biochemistry and Molecular Biology, 91, 285–288.
Guzelogli Kayisli, O., Kayisli, U. A., Luleci, G., & Arici, A. (2004). In vivo and in vitro regulation of Akt activation in human endometrial cells is estrogen dependent. Biology of Reproduction, 2004(71), 714–721.
Bain, S. D., Bailey, M. C., Celino, D. L., Lantry, M. M., & Edwards, M. W. (1993). High-dose estrogen inhibits bone resorption and stimulates bone formation in the ovariectomized mouse. Journal of Bone and Mineral Research, 8, 435–442.
Jeong, B. C., Kim, H. J., Bae, I. H., Lee, K. N., Lee, K. Y., Oh, W. M., et al. (2010). COMP-Ang1, a chimeric form of Angiopoietin 1, enhances BMP2-induced osteoblast differentiation and bone formation. Bone, 46, 479–486.
Acknowledgments
This work was supported by National Natural Science Foundation of China (NSFC, No.81372015), Shanghai Natural Science Foundation (No. 11ZR1434100) and Shanghai Municipal Health Bureau Project (No. 2013214).
Conflict of interest
The authors have no conflict of interests to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, X., Tu, Y., Zhang, L. et al. Prolyl Hydroxylase Inhibitors Protect from the Bone Loss in Ovariectomy Rats by Increasing Bone Vascularity. Cell Biochem Biophys 69, 141–149 (2014). https://doi.org/10.1007/s12013-013-9780-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-013-9780-8