Abstract
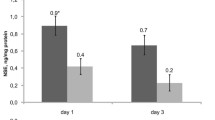
The aim of this study was to observe the dynamic changes of serum brain-derived neurotrophic factor (BDNF), S-100B, and Tau proteins levels in full-term newborns with hypoxic–ischemic encephalopathy (HIE) and to discuss their significance in brain damage. Serum samples of 28 full-term newborns diagnosed with HIE and 20 controls were obtained in the first 24 h of life. Another serum samples were also taken, respectively, at 3 and 7 days of life in HIE group. The concentrations of BDNF, S-100B, and Tau proteins were measured by the enzyme-linked immunosorbent assay method. Mean concentrations of BDNF, S-100B, and Tau proteins among different time period and in different grades of HIE group were calculated and compared. Compared with the control group, serum BDNF and proteins S-100B levels in HIE group were significantly elevated in 24 h after birth (P < 0.05) and their concentrations were also significantly higher among patients with mod-severe HIE compared to those with mild HIE at 24 h and 7 days after asphyxia (P < 0.05). Regardless of whether mod-severe HIE or mild HIE, there were no significant difference of serum BDNF and proteins S-100B among the three different time periods. There was no difference in Tau protein levels between HIE group and control group, also no difference between mod-severe HIE group and mild HIE group. BDNF and proteins S-100B are up-regulated early in asphyxia neonates with HIE; and the released amount of BDNF and proteins S-100B from nerve center system correlate with the extent of encephalopathy.



Similar content being viewed by others
References
Dixon, G., Badawi, N., Kurinczuk, J. J., Keogh, J. M., Silburn, S. R., Zubrick, S. R., et al. (2002). Early developmental outcomes after newborn encephalopathy. Pediatrics, 109(1), 26–33.
Graham, E. M., Ruis, K. A., Hartman, A. L., Northington, F. J., & Fox, H. E. (2008). A systematic review of the role of intrapartum hypoxia-ischemia in the causation of neonatal encephalopathy. American Journal of Obstetrics and Gynecology, 199(6), 587–595.
Vannucci, R. C., & Perlman, J. M. (1997). Interventions for perinatal hypoxic–ischemic encephalopathy. Pediatrics, 100(6), 1004–1014.
Walton, M., Connor, B., Lawlor, P., Young, D., Sirimanne, E., Gluckman, P., et al. (1999). Neuronal death and survival in two models of hypoxic–ischemic brain damage. Brain Research. Brain Research Reviews, 29, 137–168.
Han, B. H., & Holtzman, D. M. (2000). BDNF protects the neonatal brain from hypoxic–ischemic injury in vivo via the ERK pathway. Journal of Neuroscience, 20(15), 5775–57815.
Almli, C. R., Levy, T. J., Han, B. H., Shah, A. R., Gidday, J. M., & Holtzman, D. M. (2000). BDNF protects against spatial memory deficits following neonatal hypoxia-ischemia. Experimental Neurology, 166(1), 99–114.
Imam, S. S., Gad, G. I., Atef, S. H., & Shawky, M. A. (2009). Cord blood brain neurotrophic factor: Diagnostic and prognostic marker in fullterm newborns with perinatal asphyxia. Pakistan Journal of Biological Sciences, 12(23), 1498–1509.
Korfias, S., Stranjalis, G., Papadimitriou, A., Psachoulia, C., Daskalakis, G., Antsaklis, A., et al. (2006). Serum S-100B protein as a biochemical marker of brain injury: A review of current concepts. Current Medicinal Chemistry, 13(30), 3719–3731.
Hesse, C., Rosengren, L., Andreasen, N., Davidsson, P., Vanderstichele, H., Vanmechelen, E., et al. (2001). Transient increase in total tau but not phosphotau in human cerebrospinal fluid after acute stroke. Neuroscience Letters, 297(3), 187–190.
The Group of Neonatology, Chinese Pediatric Society, Chinese Medical Association. (2005). Diagnostic criteria for neonatal hypoxic–ischemic encephalopathy. Chinese Journal of Pediatrics, 43(8), 584–585.
Samat, H. B. & Sarnat, M. S. (1976). Neonatal encephalopathy following fetal distress. A clinical and electroencephalographic study. Archives of Neurology, 33(10), 696–705.
Bao, X. (2011). Neonatal behavioral psychology. Shao X., Ye H., & Qiu X (Eds.). Practice of Neonatology, 4th ed. Beijing: People’s Medical Publishing House (PMPH), pp. 82–86.
Acheson, A., Conover, J. C., Fandl, J. P., DeChiara, T. M., Russell, M., Thadani, A., et al. (1995). A BDNF autocrine loop in adult sensory neurons prevents cell death. Nature, 374(6521), 450–453.
Huang, E. J., & Reichardt, L. F. (2001). Neurotrophins: Roles in neuronal development and function. Annual Review of Neuroscience, 24, 677–736.
Yamada, K., & Nabeshima, T. (2003). Brain-derived neurotrophic factor/TrkB signaling in memory processes. Journal of Pharmacological Sciences, 91(4), 267–270.
Bekinschtein, P., Cammarota, M., Katche, C., Slipczuk, L., Rossato, J. I., Goldin, A., et al. (2008). BDNF is essential to promote persistence of long-term memory storage. Proceedings of the National Academy of Sciences USA, 105(7), 2711–2716.
Patapoutian, A., & Reichardt, L. F. (2001). Trk receptors: mediators of neurotrophin action. Current Opinion in Neurobiology, 11(3), 272–280.
Fernandes, C. C., Pinto-Duarte, A., Ribeiro, J. A., & Sebastião, A. M. (2008). Postsynaptic action of brain-derived neurotrophic factor attenuates alpha7 nicotinic acetylcholine receptor-mediated responses in hippocampal interneurons. Journal of Neuroscience, 28(21), 5611–5618.
Meng, M., Zhiling, W., Hui, Z., Shengfu, L., Dan, Y., & Jiping, H. (2005). Cellular levels of TrkB and MAPK in the neuroprotective role of BDNF for embryonic rat cortical neurons against hypoxia in vitro. International Journal of Developmental Neuroscience, 23(6), 515–521.
Korhonen, L., Riikonen, R., Nawa, H., & Lindholm, D. (1998). Brain derived neurotrophic factor is increased in cerebrospinal fluid of children suffering from asphyxia. Neuroscience Letters, 240(3), 151–154.
Fleiss, B., Coleman, H. A., Castillo-Melendez, M., Ireland, Z., Walker, D. W., & Parkington, H. C. (2011). Effects of birth asphyxia on neonatal hippocampal structure and function in the spiny mouse. International Journal of Developmental Neuroscience, 297, 757–766.
Riikonen, R. S., Korhonen, L. T., & Lindholm, D. B. (1999). Cerebrospinal nerve growth factor: A marker of asphyxia? Pediatric Neurology, 20(2), 137–141.
Karege, F., Schwald, M., & Cisse, M. (2002). Postnatal development profile of brain derived neurotrophic factor in rat’s brain and platelets. Neuroscience Letters, 2002(328), 262–264.
Nikolaou, K. E., Malamitsi-Puchner, A., Boutsikou, T., Economou, E., Boutsikou, M., Puchner, K. P., et al. (2006). The varying patterns of neurotrophin changes in the perinatal period. Annals of the New York Academy of Sciences, 1092, 426–433.
Blanquet, P. R., Mariani, J., & Derer, P. (2003). A calcium/calmodulin kinase pathway connects brain-derived neurotrophic factor to the cycli camp responsive transcription factor in the rat hippocampus. Neuroscience, 118(2), 477–490.
Tremblay, R., Hewitt, K., Lesiuk, H., Mealing, G., Morley, P., & Durkin, J. P. (1999). Evidence that brain-derived neurotrophic factor neuro protection is linked to it’s ability to reverse the NMDA-induced inactivation of protein kinas C in cortical neurons. Journal of Neurochemistry, 72(1), 102–111.
Bertsch, T., Casarin, W., Kretschmar, M., Zimmer, W., Walter, S., Sommer, C., et al. (2001). Protein S-100B: A serum marker for ischemic and infectious injury of cerebral tissue. Clinical Chemistry and Laboratory Medicine, 39(4), 319–323.
Bitsch, A., Horn, C., Kemmling, Y., Seipelt, M., Hellenbrand, U., Stiefel, M., et al. (2002). Serum tau protein level as a marker of axonal damage in acute ischemic stroke. European Neurology, 47(1), 45–51.
Okumus, N., Turkyilmaz, C., Onal, E. E., Atalay, Y., Serdaroglu, A., Elbeg, S., et al. (2008). Tau and S-100B proteins as biochemical markers of bilirubin-induced neurotoxicity in term neonates. Pediatric Neurology, 39(4), 245–351.
Acknowledgments
We thank XU Zheng, Vice Director, Laboratorial Center for Clinical Research, Bethune International Peace Hospital, for his assistance. We are indebted to have used the funds from Hebei Province Population and Family Planning Commission of Science and Technology Research Program (2010-A24).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Liu, F., Yang, S., Du, Z. et al. Dynamic Changes of Cerebral-Specific Proteins in Full-Term Newborns with Hypoxic–Ischemic Encephalopathy. Cell Biochem Biophys 66, 389–396 (2013). https://doi.org/10.1007/s12013-012-9478-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-012-9478-3