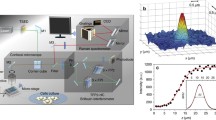
Abstract
The dynamic micromechanical and structural properties of single human red blood cells are studied using a combination of dual trap optical tweezers and confocal Raman spectroscopy. Such a combination permits us to show a direct relationship between the rheological properties and chemical structure conformation. The frequency dependence of the complex stiffness of the cells was measured using both one and two probe response functions under identical experimental conditions. Both the microrheology and Raman measurements were performed at different stretching forces applied to the cell. A detailed analysis of the auto- and cross-correlated probe motions allows exploring the local and overall viscoelastic properties of the cells over a controlled range of the deformations. The observed growth of the cell viscoelasticity with stretching was associated with structural changes in the cell membrane monitored via the Raman spectroscopy.






Similar content being viewed by others
References
Amin, S., Park, Y. K., Lue, N., Dasari, R. R., Badizadegan, K., Feld, M. S., et al. (2007). Microrheology of red blood cell membranes using dynamic scattering microscopy. Optics Express, 15, 17001–17009.
Atakhorrami, M., Mizuno, D., Koenderink, G. H., Liverpool, T. B., MacKintosh, F. C., & Schmidt, C. F. (2008). Short-time inertial response of viscoelastic fluids measured with Brownian motion and with active probes. Physical Review E, 77, 061508.
Atakhorrami, M., Sulkowska, J. I., Addas, K. M., Koenderink, G. H., Tang, J. X., Levine, A. J., et al. (2006). Correlated fluctuations of microparticles in viscoelastic solutions: Quantitative measurement of material properties by microrheology in the presence of optical traps. Physical Review E, 73, 061501.
Bankapur, A., Zachariah, E., Chidangil, S., Valiathan, M., & Mathur, D. (2010). Raman tweezers spectroscopy of live, single red and white blood cells. PLoS ONE, 5, (e10427.
Bao, G., & Suresh, S. (2003). Cell and molecular mechanics of biological materials. Nature Materials, 2, 715–725.
Berg-Sørensen, K., & Flyvbjerg, H. (2004). Power spectrum analysis for optical tweezers. Review of Scientific Instruments, 75, 594–612.
Betz, T., Lenz, M., Joanny, J. F., & Sykes, C. (2009). ATP-dependent mechanics of red blood cells. Proceedings of the National Academy of Sciences of the United States of America, 106, 15312–15317.
Brau, R. R., Ferrer, J. M., Lee, H., Castro, C. E., Tam, B. K., Tarsa, P. B., et al. (2007). Passive and active microrheology with optical tweezers. Journal of Optics A: Pure and Applied Optics, 9, S103–S112.
Brouhard, G. J., III, H. T. S., & Hunt, A. J. (2003). advanced optical tweezers for the study of cellular and molecular biomechanics. IEEE Transactions on Biomedical Engineering, 50, 121–125.
Brunner, H., & Sussner, H. (1973). Resonanse Raman scattering of haemoglobin. Biochimica et Biophysica Acta, 310, 20–31.
Chakrabarti, A., Datta, P., Bhattacharya, D., Basu S., & Saha, S. (2008). Oxidative crosslinking, spectrin and membrane interactions of hemoglobin mixtures in HbEbeta-thalassemia. Hematology, 13, 361–368.
Chan, J., Fore, S., Wachsmann-Hogiu, S., & Huser, T. (2008). Raman spectroscopy and microscopy of individual cells and cellular components. Lasers and Photonics Review 2, 325–349.
Chien, S. (1987). Red cell deformability and its relevance to blood flow.Annual Review of Physiology, 49, 177–192.
Creely, C.M., Singh, G.P., & Petrov, D. (2005). Dual wavelength optical tweezers for confocal Raman spectroscopy. Optics Communications, 245, 465–470.
Dao, M., Lim, C., & Suresh, S. (2003). Mechanics of the human red blood cell deformed by optical tweezers. Journal of Mechanics and Physics of Solids, 51, 2259–2280
Evans, E. A. (1973). New membrane concept applied to the analysis of fluid shear- and micropipette-deformed red blood cells. Biophysical Journal, 13, 941–954.
Fischer, S., Nagel, R., Bookchin, R., Jr., E. R., & Tellez-Nagel, I. (1975). The binding of hemoglobin to membranes of normal and sickle erythrocytes. Biochimica et Biophysica Acta, 375, 422–433.
Fontes, A., Castro, M. L. B., Brandao, M. M., Fernandes, H. P., Thomaz, A. A., Huruta, R. R., et al. (2011). Mechanical and electrical properties of red blood cells using optical tweezers. Journal of Optics, 13, 044012
Gallet, F., Arcizet, D., Bohec, P., & Richert, A. (2009). Power spectrum of out-of-equilibrium forces in living cells: Amplitude and frequency dependence. Soft Matter, 5, 2947–2953.
Gittes, F., & Schmidt, C. F. (1998). Interference model for back-focal-plane displacement detection in optical tweezers. Optics Letters, 23, 7–9.
Goheen, S. C., Lis, L. J., Kucuk, O., Westerman, M. P., & Kaufman, J.W. (1993). Compositional dependence of spectral features in the Raman spectra of erythrocyte membranes. Journal of Raman Spectroscopy, 24, 275–279
Gov, N. S. (2007). Active elastic network: Cytoskeleton of the red blood cell. Physical Review E, 75, 011921.
Guido, S., & Tomaiuolo, G. (2009). Microconfined flow behavior of red blood cells in vitro. Comptes Rendus Physique, 10, 751–763.
Henon, S., Lenormand, G., Richert, A., & Gallet, F. (1999). A new determination of the shear modulus of the human erythrocyte membrane using optical tweezer. Biophysical Journal, 76, 1145–1151.
Hough, L. A., & Ou-Yang, H. D. (2002). Correlated motions of two hydrodynamically coupled particles confined in separate quadratic potential wells. Physical Review E, 65, 021906.
Hu, S., Smith, K. M., & Spiro, T. G. (1996). Assignment of protoheme resonance Raman spectrum by heme labeling in myoglobin. Journal of the American Chemical Society, 118, 12638–12646.
Jay, A., & Canham, P. (1977). Viscoelastic properties of the human red blood cell membrane. II. Area and volume of individual red cells entering a micropipette. Biophysical Journal, 17, 169–178.
Lenormand, G., Henon, S., Richert, A., Simeon, J., & Gallet, F. (2001). Direct measurement of the area expansion and shear moduli of the human red blood cell membrane skeleton. Biophysical Journal, 81, 43–56.
Lippert, J. L., Gorczyca, L. E., & Meiklejohn, G. (1975). A laser Raman spectroscopic investigation of phospholipid and protein configurations in hemoglobin-free erythrocyte ghosts. Biochimica et Biophysica Acta, 382, 51–57.
Liu, R., Zheng, L., Matthews, D. L., Satake, N.,& Chan, J. W. (2011). Power dependent oxygenation state transition of red blood cells in a single beam optical trap. Applied Physics Letters, 99, 043702.
Liu, S. C., & Palek, J. (1984). Hemoglobin enhances the self-association of spectrin heterodimers in human erythrocytes. Journal of Biological Chemistry, 259, 11556–11562.
Liu, Y., Cheng, D. K., Sonek, G. J., Berns, M. W., Chapman, C. F., & Tromberg, B. J. (1995). Evidence for localized cell heating induced by infrared optical tweezers. Biophysical Journal, 68, 2137–2144.
Martinez, I. A., & Petrov, D. (2012). Force mapping of an optical trap using an acousto-optical deflector in a time-sharing regime. Applied Optics, 51, 5522–5526.
Meier, R. J. (2005). On art and science in curve-fitting vibrational spectra. Vibrational Spectroscopy, 39, 266–269.
Mills, J. P., Qie, L., Dao, M., Lim, C. T., & Suresh, S. (2004). Nonlinear elastic and viscoelastic deformation of the human red blood cell with optical tweezers. Mechanics and Chemistry of Biosystems, 1, 169–180.
Mizuno, D., Bacabac, R., Tardin, C., Head, D., Schmidt, Ch. F., & H.R.P.o.C.F.T. (2009). High-resolution probing of cellular force transmission. Physical Review Letters, 102, 168102.
Mizuno, D., Head, D. A., MacKintosh, F. C., & Schmidt, C. F. (2008). Active and passive microrheology in equilibrium and nonequilibrium systems. Macromolecules, 41, 7194–7202.
de Morales-Marinkovic, M. P., Turner, K. T., Butler, J. P., Fredberg, J. J., & Suresh, S. (2007). Viscoelasticity of the human red blood cell. American Journal of Physiology: Cell Physiology, 293, 597–605.
Neuman, K. C., & Nagy, A. (2008). Single-molecule force spectroscopy: Optical tweezers, magnetic tweezers and atomic force microscopy. Nature Methods, 5, 491–505.
Petrov, D. (2007). Raman spectroscopy of optically trapped particles. Journal of Optics A: Pure and Applied Optics, 9, S139–S156.
Rao, S., Balint, S., Cossins, B., Guallar, V., & Petrov, D. (2009). Raman study of mechanically induced oxygenation state transition of red blood cells using optical tweezers. Biophysical Journal, 96, 209–216.
Rao, S., Raj, S., Balint, S., Fons, C. B., Campoy, S., Llagostera, M., et al. (2010). Single dna molecule detection in an optical trap using surface-enhanced Raman scattering.Applied Physics Letters, 96, 213701.
Reinsch, C. H. (1967) . Smoothing by spline functions. Numerische Mathematic, 10, 177–183.
Shaklai, N., Yguerabide, J., & Ranney, H. (1977). Interaction of hemoglobin with red blood cell membranes as shown by a fluorescent chromophore. Biochemistry, 16, 5585–5592.
Snook, R. D., Harvey, T. J., Faria, E. C., & Gardner, P. (2009). Raman tweezers and their application to the study of singly trapped eukaryotic cells. Integrative Biology, 1, 43–52.
Starrs, L., & Bartlett, P. (2003). Colloidal dynamics in polymer solutions: Optical two-point microrheology measurements. Faraday Discussions, 123, 323–334.
Suresh, S. (2006). Mechanical response of human red blood cells in health and disease: Some structure-property-function relationships. Journal of Materials Research, 21, 1871–1877.
Svoboda, K., & Block, S. M. (1994). Biological applications of optical forces. Annual Review of Biophysics and Biomolecular Structure, 23, 247–285.
Tozeren, A., Skalak, R., Sung, K.L.P., & Chien, S. (1982). Viscoelastic behavior of erythrocyte membrane. Biophysical Journal, 39, 23–32.
Walder, J. A., Chatterjee, R., Steck, T. L., Low, P.S., Musso, G. F., Kaiser, E. T., et al. (1984). The interaction of hemoglobin with the cytoplasmic domain of band 3 of the human erythrocyte membrane. Journal of Biological Chemistry, 259, 10238–10246.
Wallach, D. F. H., & Verma, S. P. (1975). Raman and resonance-Raman scattering by erythrocyte ghosts. Biochimica et Biophysica Acta, 382, 542–551.
Wood, B. R., Caspers, P., Puppels, G. J., Pandiancherri, S., & McNaughton, D. (2007). Resonance Raman spectroscopy of red blood cells using near-infrared laser excitation. Analytical and Bioanalytical Chemistry, 387, 1691–1703.
Wood, B.R., & McNaughton, D. (2002). Raman excitation wavelength investigation of single red blood cells in vivo. Journal of Raman Spectroscopy, 33, 517–523.
Wood, B. R., Tait, B., & McNaughton, D. (2001). Micro-Raman characterisation of the R to T state transition of haemoglobin within a single living erythrocyte. Biochimica et Biophysica Acta, 1539, 58–70
Yan, X., Dong, R., Zhang, L., Zhang, X., & Zhang, Z. (2005). Raman spectra of single cell from gastrointestinal cancer patients. World Journal of Gastroenterology, 11, 3290–3292.
Yoon, Y. Z., Kotar, J., Brown, A. T., & Cicuta, P. (2011). Red blood cell dynamics: From spontaneous fluctuations to non-linear response. Soft Matter, 7, 2042–2051.
Yoon, Y. Z., Kotar, J., Yoon, G., & Cicuta, P. (2008). Non-linear mechanical response of the red blood cell. Physical Biology, 5, 036007.
Acknowledgments
We acknowledge the financial support from MIIN FIS2008-00114 and FIS2011-24409, (Spain), Fundació Privada Cellex Barcelona, and discussions with S. Rao, M. Marro, F. Beunis, S. Campoy.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
12013_2012_9439_MOESM1_ESM.eps
(Colour online). PSD of the single particle fluctuations (right trap) in the absence (black) andpresence of RBC and different forces applied to the cell (red—the cell at rest and blue—the cell stretched by16%). The spectrum obtained in the absence of RBC is fitted to the Lorentzian function (solid pink) and wasused for calibration purposes as described in the text. The inset shows the ratio of the PSD of the bead in thepresence of RBC to the PSD of the bead in the absence of RBC for 12 measured cells, demonstrating that thelow frequency effect is characteristic for all studied cells. The ratio exceeds unity for most of the cells at lowfrequency (EPS 223 KB)
12013_2012_9439_MOESM2_ESM.eps
Representative PSD of water (black) and Alsever’s (red) solution showing asmall difference due to the variation in the viscosity of these solutions. The experiments were performedunder the same conditions (excluding the bead polydispersity) using the beads from the same batch. Thesolid green line represents the Lorentzian fitting of the PSD in Alsever’s solution. As expected, the higherviscosity of Alsever’s solution leads to a lowering of the corner frequency (in this case from 59 Hz to 53Hz) and slightly higher PSD values below the corner frequency. The final value of the viscosity of Alsever’ssolution was found as an average value obtained from 40 PSD spectra for each solution (EPS 1258 KB)
12013_2012_9439_MOESM3_ESM.eps
Power spectral density of the relative fluctuations of the beads in the absence(black), r = 9.2 μm and presence of RBC and different forces applied to the cell (red circles—the cell at rest,r0 = 9.8 μm, green (right) triangles—the cell stretched by 12%, r1 = 10.6 μm, blue (up) triangles—the cellstretched by 16% , r2 = 10.8 μm). In the presence of RBC, PSDδ_x represents the PSD corresponding tothe fluctuations of the cell length; r is the centre-to-centre distance between the beads (EPS 132 KB)
12013_2012_9439_MOESM4_ESM.eps
Frequency dependence of the real (a) and imaginary (b) parts of an apparentsingle-particle response function (right trap) in the absence (black squares) and presence of RBC and differentforces applied to the cell (red circles - the cell at rest, green (right) triangles - the cell stretched by 12%, andblue (up) triangles - the cell stretched by 16%) (EPS 1212 KB)
12013_2012_9439_MOESM5_ESM.eps
Real part of the complex stiffness as a function of frequency measured viathe single particle (right trap) rheology (red circles—cell at rest, green (right) triangles—stretched by 12%,and blue (up) triangles—stretched by 16%). The actual complex stiffness was calculated using the responsefunctions corrected for the presence of two traps (EPS 1099 KB)
12013_2012_9439_MOESM6_ESM.eps
Imaginary part of the complex stiffness measured with the two particle microrheologyfor the cell at rest (red circles, r0 = 9.8 μm) and stretched by 12% (green (right) triangles,r1 = 10.6 μm) and 16% (blue (up) triangles, r2 = 10.8 μm); r is the centre-to-centre distance between thebeads (EPS 1102 KB)
12013_2012_9439_MOESM8_ESM.eps
Raman spectra intensity of 991 cm−1, 1442 cm−1 and 1530 cm−1 bands of single RBC plottedagainst the relative deformation (EPS 44 KB)
Rights and permissions
About this article
Cite this article
Raj, S., Wojdyla, M. & Petrov, D. Studying Single Red Blood Cells Under a Tunable External Force by Combining Passive Microrheology with Raman Spectroscopy. Cell Biochem Biophys 65, 347–361 (2013). https://doi.org/10.1007/s12013-012-9439-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-012-9439-x