Abstract
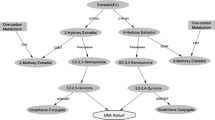
The aim of this case–control study is to explore the role of aberrations in xenobiotic metabolism in inducing oxidative DNA damage and altering the susceptibility to breast cancer. Cytochrome P4501A1 (CYP1A1) m1 (OR: 1.41, 95% CI 1.08–1.84), CYP1A1 m4 (OR: 5.13, 95% CI 2.68–9.81), Catecholamine-O-methyl transferase (COMT) H108L (OR: 1.49, 95% CI 1.16–1.92), and glutathione S-transferase (GST) T1 null (OR: 1.68, 95% CI 1.09–2.59) variants showed association with breast cancer risk. Reduced folate carrier 1 (RFC1) 80A/CYP1A1 m1/CYP1A1 m4 and RFC1 80A/thymidylate synthase (TYMS) 5′-UTR 2R/methionine synthase (MTR) 2756G/COMT 108L genetic combinations were found to inflate breast cancer risk under the conditions of low dietary folate (345 ± 110 vs. 379 ± 139 μg/day) and low plasma folate (6.81 ± 1.25 vs. 7.09 ± 1.26 ng/ml) by increasing plasma 8-oxo-2′-deoxyguanosine (8-oxodG). This increase in 8-oxodG is attributed to low methionine (49.38 ± 23.74 vs. 53.90 ± 23.85 μmol/l); low glutathione (378 ± 242 vs. 501 ± 126 μmol/l) and GSTT1 null variant; and hypermethylation of CpG island of extracellular-superoxide dismutase (EC-SOD) (92.78 ± 11.49 vs. 80.45 ± 9.86%), which impair O-methylation of catechol estrogens to methoxy estrogens, conjugation of glutathione to semiquinones/quinones and free radical scavenging respectively. Our results suggest cross-talk between one-carbon metabolism and xenobiotic metabolism influencing oxidative DNA damage and susceptibility to breast cancer.


Similar content being viewed by others
References
Jemal, A., Bray, F., Center, M. M., Ferlay, J., Ward, E., & Forman, D. (2011). Global cancer statistics. CA: A Cancer Journal for Clinicians, 61(2), 69–90.
Scheuner, M. T., McNeel, T. S., & Freedman, A. N. (2010). Population prevalence of familial cancer and common hereditary cancer syndromes. The 2005 California Health Interview Survey. Genetics in Medicine, 12(11), 726–735.
Kabuto, M., Akiba, S., Stevens, R. G., Neriishi, K., & Land, C. E. (2000). A prospective study of estradiol and breast cancer in Japanese women. Cancer Epidemiology, Biomarkers and Prevention, 9(6), 575–579.
Weichselbaum, R. R., Hellman, S., Piro, A. J., Nove, J. J., & Little, J. B. (1978). Proliferation kinetics of a human breast cancer line in vitro following treatment with 17beta-estradiol and 1-beta-d-arabinofuranosylcytosine. Cancer Research, 38(8), 2339–2342.
Wang, Z., Wijewickrama, G. T., Peng, K. W., Dietz, B. M., Yuan, L., van Breemen, R. B., et al. (2009). Estrogen receptor alpha enhances the rate of oxidative DNA damage by targeting an equine estrogen catechol metabolite to the nucleus. Journal of Biological Chemistry, 284(13), 8633–8642.
Hayes, C. L., Spink, D. C., Spink, B. C., Cao, J. Q., Walker, N. J., & Sutter, T. R. (1996). 17 beta-estradiol hydroxylation catalyzed by human cytochrome P450 1B1. Proc Natl Acad Sci USA, 93(18), 9776–9781.
Spink, D. C., Spink, B. C., Cao, J. Q., DePasquale, J. A., Pentecost, B. T., Fasco, M. J., et al. (1998). Differential expression of CYP1A1 and CYP1B1 in human breast epithelial cells and breast tumor cells. Carcinogenesis, 19(2), 291–298.
Männistö, P. T., & Kaakkola, S. (1999). Catechol-O-methyltransferase (COMT): Biochemistry, molecular biology, pharmacology, and clinical efficacy of the new selective COMT inhibitors. Pharmacological Reviews, 51(4), 593–628.
Takahashi, N., Schreiber, J., Fischer, V., & Mason, R. P. (1987). Formation of glutathione-conjugated semiquinones by the reaction of quinones with glutathione: An ESR study. Archives of Biochemistry and Biophysics, 252(1), 41–48.
Kawajiri, K., Nakachi, K., Imai, K., Shinoda, N., & Watanabe, J. (1990). Identification of genetically high risk individuals to lung cancer by DNA polymorphisms of the cytochrome P4SOIAI gene. FEBS Letters, 263, 131–133.
Hayashi, S., Watanabe, J., Nakachi, K., & Kawajiri, K. (1991). Genetic linkage of lung cancer-associated MspI polymorphism with amino acid replacement in the heme binding region of the human cytochrome P4501A1 gene. Journal of Biochemistry, 110, 407–411.
Cascorbi, I., Brockmoller, J., & Roots, I. (1996). A C4887A polymorphism in exon 7 of human CYPIAI: Population frequency, mutation linkages, and impact on lung cancer susceptibility. Cancer Research, 56, 4965–4969.
Landi, M. T., Bertazzi, P. A., Shields, P. G., Clark, G., Lucier, G. W., Garte, S. J., et al. (1994). Association between CYP1A1 genotype, mRNA expression and enzymatic activity in humans. Pharmacogenetics, 4(5), 242–246.
Moysich, K. B., Shields, P. G., Freudenheim, J. L., Schisterman, E. F., Vena, J. E., Kostyniak, P., et al. (1999). Polychlorinated biphenyls, cytochrome P4501A1 polymorphism, and postmenopausal breast cancer risk. Cancer Epidemiology, Biomarkers and Prevention, 8(1), 41–44.
Bailey, L. R., Roodi, N., Verrier, C. S., Yee, C. J., Dupont, W. D., & Parl, F. F. (1998). Breast cancer and CYPIA1, GSTM1, and GSTT1 polymorphisms: Evidence of a lack of association in Caucasians and African Americans. Cancer Research, 58(1), 65.
Firozi, P. F., Bondy, M. L., Sahin, A. A., Chang, P., Lukmanji, F., Singletary, E. S., et al. (2002). Aromatic DNA adducts and polymorphisms of CYP1A1, NAT2, and GSTM1 in breast cancer. Carcinogenesis, 23(2), 301–306.
Masson, L. F., Sharp, L., Cotton, S. C., & Little, J. (2005). Cytochrome P-450 1A1 gene polymorphisms and risk of breast cancer: A HuGE review. American Journal of Epidemiology, 161(10), 901–915.
Doyle, A. E., & Yager, J. D. (2008). Catechol-O-methyltransferase: Effects of the val108met polymorphism on protein turnover in human cells. Biochimica et Biophysica Acta, 1780(1), 27–33.
Zahid, M., Saeed, M., Lu, F., Gaikwad, N., Rogan, E., & Cavalieri, E. (2007). Inhibition of catechol-O-methyltransferase increases estrogen-DNA adduct formation. Free Radical Biology and Medicine, 43(11), 1534–1540.
Wang, Q., Wang, Y. P., Li, J. Y., Yuan, P., Yang, F., & Li, H. (2010). Polymorphic catechol-O-methyltransferase gene, soy isoflavone intake and breast cancer in postmenopausal women: A case–control study. Chinese Journal of Cancer, 29(7), 683–688.
Mao, C., Wang, X. W., Qiu, L. X., Liao, R. Y., Ding, H., & Chen, Q. (2010). Lack of association between catechol-O-methyltransferase Val108/158Met polymorphism and breast cancer risk: A meta-analysis of 25,627 cases and 34,222 controls. Breast Cancer Research and Treatment, 121(3), 719–725.
Sergentanis, T. N., & Economopoulos, K. P. (2010). GSTT1 and GSTP1 polymorphisms and breast cancer risk: A meta-analysis. Breast Cancer Research and Treatment, 121(1), 195–202.
Cribb, A. E., Joy Knight, M., Guernsey, J., Dryer, D., Hender, K., Shawwa, A., et al. (2011). CYP17, catechol-o-methyltransferase, and glutathione transferase M1 genetic polymorphisms, lifestyle factors, and breast cancer risk in women on Prince Edward Island. Breast Journal, 17(1), 24–31.
Naushad, S. M., Pavani, A., Digumarti, R. R., Gottumukkala, S. R., & Kutala, V. K. (2010). Epistatic interactions between loci of one-carbon metabolism modulate susceptibility to breast cancer. Molecular Biology Reports [Epub ahead of print].
Mohammad, N. S., Yedluri, R., Addepalli, P., Gottumukkala, S. R., Digumarti, R. R., & Kutala, V. K. (2011). Aberrations in one-carbon metabolism induce oxidative DNA damage in sporadic breast cancer. Molecular and Cellular Biochemistry, 349(1–2), 159–167.
Ellman, G. L. (1959). Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics, 82(1), 70–77.
Radha Rama Devi, A., Naushad, S. M., & Prasad, K. C. (2006). Evaluation of total plasma homocysteine in Indian newborns using heel-prick samples. Indian Journal of Pediatrics, 73(6), 503–508.
McCord, J. M., & Fridovich, I. (1969). Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). Journal of Biological Chemistry, 244(2), 6049–6055.
Shin, A., Kang, D., Choi, J. Y., Lee, K. M., Park, S. K., Noh, D. Y., et al. (2007). Cytochrome P450 1A1 (CYP1A1) polymorphisms and breast cancer risk in Korean women. Experimental and Molecular Medicine, 39(3), 361–366.
Syamala, V. S., Syamala, V., Sheeja, V. R., Kuttan, R., Balakrishnan, R., & Ankathil, R. (2010). Possible risk modification by polymorphisms of estrogen metabolizing genes in familial breast cancer susceptibility in an Indian population. Cancer Investigation, 28(3), 304–311.
Basham, V. M., Pharoah, P. D., Healey, C. S., Luben, R. N., Day, N. E., Easton, D. F., et al. (2001). Polymorphisms in CYP1A1 and smoking: No association with breast cancer risk. Carcinogenesis, 22(11), 1797–1800.
Singh, V., Rastogi, N., Sinha, A., Kumar, A., Mathur, N., & Singh, M. P. (2007). A study on the association of cytochrome-P450 1A1 polymorphism and breast cancer risk in north Indian women. Breast Cancer Research and Treatment, 101(1), 73–81.
Okobia, M., Bunker, C., Zmuda, J., Kammerer, C., Vogel, V., Uche, E., et al. (2005). Cytochrome P4501A1 genetic polymorphisms and breast cancer risk in Nigerian women. Breast Cancer Research and Treatment, 94(3), 285–293.
Hamaguchi, M., Nishio, M., Toyama, T., Sugiura, H., Kondo, N., Fujii, Y., et al. (2008). Possible difference in frequencies of genetic polymorphisms of estrogen receptor alpha, estrogen metabolism and P53 genes between estrogen receptor-positive and -negative breast cancers. Japanese Journal of Clinical Oncology, 38(11), 734–742.
Gaudet, M. M., Chanock, S., Lissowska, J., Berndt, S. I., Peplonska, B., Brinton, L. A., et al. (2006). Comprehensive assessment of genetic variation of catechol-O-methyltransferase and breast cancer risk. Cancer Research, 66(19), 9781–9785.
Embrechts, J., Lemière, F., Van Dongen, W., Esmans, E. L., Buytaert, P., Van Marck, E., et al. (2003). Detection of estrogen DNA-adducts in human breast tumor tissue and healthy tissue by combined nano LC-nano ES tandem mass spectrometry. J Am Soc Mass Spectrom, 14(5), 482–491.
Govindaiah, V., Naushad, S. M., Prabhakara, K., Krishna, P. C., & Radha Rama Devi, A. (2009). Association of parental hyperhomocysteinemia and C677T methylene tetrahydrofolate reductase (MTHFR) polymorphism with recurrent pregnancy loss. Clinical Biochemistry, 42(4–5), 380–386.
Oikawa, S., Murakami, K., & Kawanishi, S. (2003). Oxidative damage to cellular and isolated DNA by homocysteine: Implications for carcinogenesis. Oncogene, 22(23), 3530–3538.
Hitchler, M. J., Wikainapakul, K., Yu, L., Powers, K., Attatippaholkun, W., & Domann, F. E. (2006). Epigenetic regulation of manganese superoxide dismutase expression in human breast cancer cells. Epigenetics, 1(4), 163–171.
Wolthers, K. R., & Scrutton, N. S. (2009). Cobalamin uptake and reactivation occurs through specific protein interactions in the methionine synthase-methionine synthase reductase complex. FEBS Journal, 276(7), 1942–1951.
Majumdar, S., Mukherjee, S., Maiti, A., Karmakar, S., Das, A. S., Mukherjee, M., et al. (2009). Folic acid or combination of folic acid and vitamin B(12) prevents short-term arsenic trioxide-induced systemic and mitochondrial dysfunction and DNA damage. Environment Toxicology, 24(4), 377–387.
Bagnyukova, T. V., Powell, C. L., Pavliv, O., Tryndyak, V. P., & Pogribny, I. P. (2008). Induction of oxidative stress and DNA damage in rat brain by a folate/methyl-deficient diet. Brain Research, 1237, 44–51.
Schmielau, J., & Finn, O. J. (2001). Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Research, 61(12), 4756–4760.
Ohba, M., Shibanuma, M., Kuroki, T., & Nose, K. (1994). Production of hydrogen peroxide by transforming growth factor-beta 1 and its involvement in induction of egr-1 in mouse osteoblastic cells. Journal of Cell Biology, 126(4), 1079–1088.
Szatrowski, T. P., & Nathan, C. F. (1991). Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Research, 51(3), 794–798.
Acknowledgments
This study was supported by the grant funded by Indian Council of Medical Research (ICMR), New Delhi (Ref No. 5/13/32/2007). Dr. V.K. Kutala is recipient of Ramanujan Fellowship awarded by Department of Science & Technology (DST), Government of India. We thank Dr. Annie Q Hasan, Head, Department of Molecular Medicine, Kamineni Hospitals, Hyderabad for critical revision of the manuscript in terms of genetic aspects.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Naushad, S.M., Reddy, C.A., Rupasree, Y. et al. Cross-Talk Between One-Carbon Metabolism and Xenobiotic Metabolism: Implications on Oxidative DNA Damage and Susceptibility to Breast Cancer. Cell Biochem Biophys 61, 715–723 (2011). https://doi.org/10.1007/s12013-011-9245-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12013-011-9245-x