Abstract
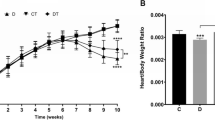
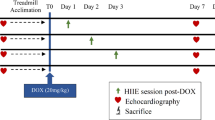
Doxorubicin (DOX) is an anticancer chemotherapy drug that is widely used in clinical practice. It is well documented that DOX impairs baroreflex responsiveness and left ventricular function and enhances sympathetic activity, cardiac sympathetic afferent reflexes and oxidative stress, which contribute to hemodynamic deterioration. Because resistance training (RT)-induced cardioprotection has been observed in other animal models, the objective of this study was to assess the effects of RT during DOX treatment on hemodynamics, arterial baroreflex, cardiac autonomic tone, left ventricular function and oxidative stress in rats with DOX-induced cardiotoxicity. Male Wistar rats were submitted to a RT protocol (3 sets of 10 repetitions, 40% of one-repetition maximum (1RM) of intensity, 3 times per week, for 8 weeks). The rats were separated into 3 groups: sedentary control, DOX sedentary (2.5 mg/kg of DOX intraperitoneal injection, once a week, for 6 weeks) and DOX + RT. After training or time control, the animals were anesthetized and 2 catheters were implanted for hemodynamic, arterial baroreflex and cardiac autonomic tone. Another group of animals was used to evaluate left ventricular function. We found that RT in DOX-treated rats decreased diastolic arterial pressure, heart rate, sympathetic tone and oxidative stress. In addition, RT increased arterial baroreflex sensitivity, vagal tone and left ventricular developed pressure in rats with DOX-induced cardiotoxicity. In summary, RT is a useful non-pharmacological strategy to attenuate DOX-induced cardiotoxicity.




Similar content being viewed by others
References
Sawyer, D. B., Peng, X., Chen, B., Pentassuglia, L., & Lim, C. C. (2010). Mechanisms of anthracycline cardiac injury: Can we identify strategies for Cardioprotection? Progress in Cardiovascular Diseases, 53(2), 105–113. https://doi.org/10.1016/j.pcad.2010.06.007.
Minotti, G., Menna, P., Salvatorelli, E., Cairo, G., & Gianni, L. (2004). Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews, 56(2), 185. https://doi.org/10.1124/pr.56.2.6.
Al-malky, H. S., Al Harthi, S. E., & Osman, A.-M. M. (2020). Major obstacles to doxorubicin therapy: Cardiotoxicity and drug resistance. Journal of Oncology Pharmacy Practice, 26(2), 434–444. https://doi.org/10.1177/1078155219877931.
Takemura, G., & Fujiwara, H. (2007). Doxorubicin-induced cardiomyopathy: From the Cardiotoxic mechanisms to management. Special Articles, 49(5), 330–352. https://doi.org/10.1016/j.pcad.2006.10.002.
Marques-Aleixo, I., Santos-Alves, E., Torrella, J. R., Oliveira, P. J., Magalhães, J., & Ascensão, A. (2018). Exercise and doxorubicin treatment modulate cardiac mitochondrial quality control signaling. Cardiovascular Toxicology, 18(1), 43–55. https://doi.org/10.1007/s12012-017-9412-4.
Hasinoff, B., Patel, D., & Wu, X. (2020). The role of topoisomerase IIβ in the mechanisms of action of the doxorubicin Cardioprotective agent Dexrazoxane. Cardiovascular Toxicology, 20(3). https://doi.org/10.1007/s12012-019-09554-5.
Songbo, M., Lang, H., Xinyong, C., Bin, X., Ping, Z., & Liang, S. (2019). Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicology Letters, 1(307), 41–48. https://doi.org/10.1016/j.toxlet.2019.02.013.
Chatterjee, K., Zhang, J., Honbo, N., & Karliner, J. S. (2010). Doxorubicin cardiomyopathy. Cardiology, 115(2), 155–162. https://doi.org/10.1159/000265166.
Dolinsky, V. W., Rogan, K. J., Sung, M. M., Zordoky, B. N., Haykowsky, M. J., Young, M. E., & Dyck, J. R. B. (2013). Both aerobic exercise and resveratrol supplementation attenuate doxorubicin-induced cardiac injury in mice. AJP: Endocrinology and Metabolism, 305(2), 243–253. https://doi.org/10.1152/ajpendo.00044.2013.
Marques-Aleixo, I., Santos-Alves, E., Mariani, D., Rizo-Roca, D., Padrão, A. I., Rocha-Rodrigues, S., & Ascensão, A. (2015). Physical exercise prior and during treatment reduces sub-chronic doxorubicin-induced mitochondrial toxicity and oxidative stress. Mitochondrion, 20, 22–33. https://doi.org/10.1016/j.mito.2014.10.008.
Zolfagharzadeh, F., & Roshan, V. D. (2013). Pretreatment hepatoprotective effect of regular aerobic training against hepatic toxicity induced by doxorubicin in rats. Asian Pacific Journal of Cancer Prevention, 14(5), 2931–2936. https://doi.org/10.7314/APJCP.2013.14.5.2931.
Zhang, S. (2012). Enhanced sympathetic activity and cardiac sympathetic afferent reflex in rats with heart failure induced by adriamycin. Journal of Biomedical Research, 26(6), 425–431. https://doi.org/10.7555/JBR.26.20120035.
Rossi, N. F., Maliszewska-Scislo, M., & Chen, H. (2008). Central endothelin: Effects on vasopressin and the arterial baroreflex in doxorubicin heart failure ratsThis article is one of a selection of papers published in the special issue (part 1 of 2) on forefronts in endothelin. Canadian Journal of Physiology and Pharmacology, 86(6), 343–352. https://doi.org/10.1139/Y08-027.
Sano, N., Way, D., & McGrath, B. P. (1990). Renal norepinephrine spillover and baroreflex responses in evolving heart failure. American Journal of Physiology - Renal Physiology, 258(6), F1516.
Chicco, A. J., Hydock, D. S., Schneider, C. M., & Hayward, R. (2006). Low-intensity exercise training during doxorubicin treatment protects against cardiotoxicity. Journal of Applied Physiology, 100(2), 519–527. https://doi.org/10.1152/japplphysiol.00148.2005.
Potočnik, N., Perše, M., Cerar, A., Injac, R., & Finderle, Ž. (2017). Cardiac autonomic modulation induced by doxorubicin in a rodent model of colorectal cancer and the influence of fullerenol pretreatment. PLoS One, 12(7), e0181632. https://doi.org/10.1371/journal.pone.0181632.
Chen, W.-W., Xiong, X.-Q., Chen, Q., Li, Y.-H., Kang, Y.-M., & Zhu, G.-Q. (2015). Cardiac sympathetic afferent reflex and its implications for sympathetic activation in chronic heart failure and hypertension. Acta Physiologica, 213(4), 778–794. https://doi.org/10.1111/apha.12447.
Koba, S. (2018). Angiotensin II, oxidative stress, and sympathetic nervous system hyperactivity in heart failure. Yonago Acta Medica, 61, 103–109.
Rabelo, E., De Angelis, K., Bock, P., Gatelli Fernandes, T., Cervo, F., Belló Klein, A., & Cláudia Irigoyen, M. (2001). Baroreflex sensitivity and oxidative stress in Adriamycin-induced heart failure. Hypertension, 38(3), 576–580. https://doi.org/10.1161/hy09t1.096185.
O’Connell, J. L., Romano, M. M. D., Campos Pulici, E. C., Carvalho, E. E. V., de Souza, F. R., Tanaka, D. M., & Simões, M. V. Short-term and long-term models of doxorubicin-induced cardiomyopathy in rats: A comparison of functional and histopathological changes. Experimental and Toxicologic Pathology. https://doi.org/10.1016/j.etp.2017.01.004.
Sturgeon, K., Muthukumaran, G., Ding, D., Bajulaiye, A., Ferrari, V., & Libonati, J. R. (2015). Moderate-intensity treadmill exercise training decreases murine cardiomyocyte cross-sectional area. Physiological Reports, 3(5), e12406–e12406. https://doi.org/10.14814/phy2.12406.
Shirinbayan, V., & Roshan, V. D. (2012). Pretreatment effect of running exercise on HSP 70 and DOX-induced cardiotoxicity. Asian Pacific Journal of Cancer Prevention, 13(11), 5849–5855. https://doi.org/10.7314/APJCP.2012.13.11.5849.
Ahmadian, M., & Dabidi Roshan, V. (2018). Modulatory effect of aerobic exercise training on doxorubicin-induced cardiotoxicity in rats with different ages. Cardiovascular Toxicology, 1, 33–42. https://doi.org/10.1007/s12012-017-9411-5.
Smuder, A. J. (2019). Exercise stimulates beneficial adaptations to diminish doxorubicin-induced cellular toxicity. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 317(5), R662–R672. https://doi.org/10.1152/ajpregu.00161.2019.
Chicco, A. J., McCarty, H., Reed, A. H., Story, R. R., Westerlind, K. C., Turner, R. T., & Hayward, R. (2006). Resistance exercise training attenuates alcohol-induced cardiac oxidative stress. European Journal of Cardiovascular Prevention & Rehabilitation, 13(1), 74–79.
Alves, J. P., Nunes, R. B., Stefani, G. P., & Dal Lago, P. (2014). Resistance training improves hemodynamic function, collagen deposition and inflammatory profiles: Experimental model of heart failure. PLoS One, 9(10), 110–317. https://doi.org/10.1371/journal.pone.0110317.
Soufi, F. G., Saber, M. M., Ghiassie, R., & Alipour, M. (2011). Role of 12-week resistance training in preserving the heart against ischemia-reperfusion-induced injury. Cardiology Journal, 18(2), 140–145.
Santana, M. N. S., Souza, D. S., Miguel-dos-Santos, R., Rabelo, T. K., de Vasconcelos, C. M. L., Navia-Pelaez, J. M., & Mesquita, T. R. R. (2018). Resistance exercise mediates remote ischemic preconditioning by limiting cardiac eNOS uncoupling. Journal of Molecular and Cellular Cardiology, 125, 61–72. https://doi.org/10.1016/j.yjmcc.2018.10.016.
Shen, Y., Park, J. B., Lee, S. Y., Han, S. K., & Ryu, P. D. (2019). Exercise training normalizes elevated firing rate of hypothalamic presympathetic neurons in heart failure rats. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 316(2), R110–R120. https://doi.org/10.1152/ajpregu.00225.2018.
Pfannenstiel, K., & Hayward, R. (2018). Effects of resistance exercise training on doxorubicin-induced cardiotoxicity. Journal of Cardiovascular Pharmacology, 1. https://doi.org/10.1097/FJC.0000000000000574.
Kerr, J., Anderson, C., & Lippman, S. M. (2017). Physical activity, sedentary behaviour, diet, and cancer: An update and emerging new evidence. The Lancet Oncology, 18(8), 457–471.
Segal, R., Zwaal, C., Green, E., Tomasone, J. R., Loblaw, A., Petrella, T., & With Cancer Guideline Development Group, T. E. for P. (2017). Exercise for people with cancer: A clinical practice guideline. Current Oncology, 24(1), 40. https://doi.org/10.3747/co.24.3376.
Tamaki, T., Uchiyama, S., & Nakano, S. (1992). A weight-lifting exercise model for inducing hypertrophy in the hindlimb muscles of rats. Medicine & Science in Sports & Exercise, 24(8).
Macedo, F. N., Mesquita, T. R. R., Melo, V. U., Mota, M. M., Silva, T. L. T. B., Santana, M. N., & Santana-Filho, V. J. (2016). Increased nitric oxide bioavailability and decreased sympathetic modulation are involved in vascular adjustments induced by low-intensity resistance training. Frontiers in Physiology, 7. https://doi.org/10.3389/fphys.2016.00265.
Bertinieri, G., Di Rienzo, M., Cavallazzi, A., Ferrari, A., Pedotti, A., & Mancia, G. (1985). A new approach to analysis of the arterial baroreflex. Journal of Hypertension Supplement: Official Journal of the International Society of Hypertension, 3(3), 79–81.
Di Rienzo, M., Parati, G., Castiglioni, P., Tordi, R., Mancia, G., & Pedotti, A. (2001). Baroreflex effectiveness index: An additional measure of baroreflex control of heart rate in daily life. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 280(3), R744–R751. https://doi.org/10.1152/ajpregu.2001.280.3.R744.
Ichige, M. H. A., Santos, C. R., Jordão, C. P., Ceroni, A., Negrão, C. E., & Michelini, L. C. (2016). Exercise training preserves vagal preganglionic neurones and restores parasympathetic tonus in heart failure: Autonomic balance: Exercise training and heart failure. The Journal of Physiology, 594(21), 6241–6254. https://doi.org/10.1113/JP272730.
de Britto, R. M., da Silva-Neto, J. A., Mesquita, T. R. R., de Vasconcelos, C. M. L., de Almeida, G. K. M., de Jesus, I. C. G., & Lauton-Santos, S. (2018). Myrtenol protects against myocardial ischemia-reperfusion injury through antioxidant and anti-apoptotic dependent mechanisms. Food and Chemical Toxicology, 111, 557–566. https://doi.org/10.1016/j.fct.2017.12.003.
Erickson, J. R., Joiner, M. A., Guan, X., Kutschke, W., Yang, J., Oddis, C. V., & Anderson, M. E. (2008). A dynamic pathway for calcium-independent activation of CaMKII by methionine oxidation. Cell, 133(3), 462–474. https://doi.org/10.1016/j.cell.2008.02.048.
Owusu-Ansah, E., Yavari, A., & Banerjee, U. (2008). A protocol for in vivo detection of reactive oxygen species. https://doi.org/10.1038/nprot.2008.23.
Festing, M. F. W. (2006). Design and statistical methods in studies using animal models of development. ILAR Journal, 47(1), 5–14. https://doi.org/10.1093/ilar.47.1.5.
Miot, H. A. (2017). Avaliação da normalidade dos dados em estudos clínicos e experimentais. Jornal Vascular Brasileiro, 16(2), 88–91. https://doi.org/10.1590/1677-5449.041117.
BASSER, R., & GREEN, M. (1993). Strategies for prevention of anthracycline cardiotoxicity. Cancer Treatment Reviews, 19(1), 57–77.
Hayward, R., & Hydock, D. S. (2007). Doxorubicin cardiotoxicity in the rat: An in vivo characterization. Journal of the American Association for Laboratory Animal Science, 46(4), 20–32.
van Dalen, E. C., van der Pal, H. J., & Kremer, L. C. (2016). Different dosage schedules for reducing cardiotoxicity in people with cancer receiving anthracycline chemotherapy. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.CD005008.pub4.
da Palma, R. K., Moraes-Silva, I. C., da Silva Dias, D., Shimojo, G. L., Conti, F. F., Bernardes, N., & De Angelis, K. (2016). Resistance or aerobic training decreases blood pressure and improves cardiovascular autonomic control and oxidative stress in hypertensive menopausal rats. Journal of Applied Physiology, 121(4), 1032–1038. https://doi.org/10.1152/japplphysiol.00130.2016.
Shimojo, G. L., Palma, R. K., Brito, J. O., Sanches, I. C., Irigoyen, M. C., & De Angelis, K. (2015). Dynamic resistance training decreases sympathetic tone in hypertensive ovariectomized rats. Brazilian Journal of Medical and Biological Research, 48(6), 523–527. https://doi.org/10.1590/1414-431X20154387.
La Rovere, M. T., Pinna, G. D., & Raczak, G. (2008). Baroreflex sensitivity: Measurement and clinical implications. Annals of Noninvasive Electrocardiology, 13(2), 191–207.
Pinna, G. D., Maestri, R., & La Rovere, M. T. (2015). Assessment of baroreflex sensitivity from spontaneous oscillations of blood pressure and heart rate: Proven clinical value? Physiological Measurement, 36(4), 741–753. https://doi.org/10.1088/0967-3334/36/4/741.
Brum, P. C., Da Silva, G. J. J., Moreira, E. D., Ida, F., Negrão, C. E., & Krieger, E. M. (2000). Exercise training increases baroreceptor gain sensitivity in Normal and hypertensive rats. Hypertension, 36, 1018–1022. https://doi.org/10.1161/01.HYP.36.6.1018.
Cavalleri, M. T., Burgi, K., Cruz, J. C., Jordão, M. T., Ceroni, A., & Michelini, L. C. (2011). Afferent signaling drives oxytocinergic preautonomic neurons and mediates training-induced plasticity. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 301(4), 958–966. https://doi.org/10.1152/ajpregu.00104.2011.
Koba, S., Hisatome, I., & Watanabe, T. (2014). Central command dysfunction in rats with heart failure is mediated by brain oxidative stress and normalized by exercise training: Autonomic dysfunction in heart failure and exercise training effects. The Journal of Physiology, 592(17), 3917–3931. https://doi.org/10.1113/jphysiol.2014.272377.
Barauna, V. G., Junior, M. L. B., Rosa, C., Casarini, D. E., Krieger, J. E., & Oliveira, E. M. (2005). Cardiovascular adaptations in rats submitted to a resistance-training model. Clinical and Experimental Pharmacology and Physiology, 32(4), 249–254.
de Cássia Cypriano Ervati Pinter, R., Padilha, A. S., de Oliveira, E. M., Vassallo, D. V., & de Fúcio Lizardo, J. H. (2008). Cardiovascular adaptive responses in rats submitted to moderate resistance training. European Journal of Applied Physiology, 103(5), 605–613. https://doi.org/10.1007/s00421-008-0761-3.
Kotamraju, S., Kalivendi, S., Konorev, E., Chitambar, C., Joseph, J., & Kalyanaraman. (2004). Oxidant-Induced Iron Signaling in Doxorubicin-Mediated Apoptosis. Methods in Enzymology, 378.
Salo, C., Pacifici, E., Lin, W., & Davies, J. A. (1990). Superoxide Dismutase Undergoes Proteolysis and Fragmentation following Oxidative Modification and Inactivation. The Journal of Biological Chemistry, 10.
Shringarpure, R., Grune, T., Mehlhase, J., & Davies, K. J. A. (2003). Ubiquitin conjugation is not required for the degradation of oxidized proteins by proteasome. Journal of Biological Chemistry, 278(1), 311–318. https://doi.org/10.1074/jbc.M206279200.
Shringarpure, R., Grune, T., & Davies, K. J. A. (2001). Protein oxidation and 20S proteasome-dependent proteolysis in mammalian cells. Cellular and Molecular Life Sciences, 58(10), 1442–1450. https://doi.org/10.1007/PL00000787.
Behonick, G. S., Novak, M. J., Nealley, E. W., & Baskin, S. I. (2001). Toxicology update: The cardiotoxicity of the oxidative stress metabolites of catecholamines (aminochromes). Journal of Applied Toxicology, 21, 15–22. https://doi.org/10.1002/jat.793.
Bertagnolli, M., Schenkel, P. C., Campos, C., Mostarda, C. T., Casarini, D. E., Bello-Klein, A., & Rigatto, K. (2008). Exercise training reduces sympathetic modulation on cardiovascular system and cardiac oxidative stress in spontaneously hypertensive rats. American Journal of Hypertension, 21(11), 1188–1193. https://doi.org/10.1038/ajh.2008.270.
Gilliam, L. A. A., & St. Clair, D. K. (2011). Chemotherapy-induced weakness and fatigue in skeletal muscle: The role of oxidative stress. Antioxidants & Redox Signaling, 15(9), 2543–2563. https://doi.org/10.1089/ars.2011.3965.
Coussens, L. M., & Werb, Z. (2002). Inflammation and cancer. Nature, 420(6917), 860–867. https://doi.org/10.1038/nature01322.
Prasad, S., Gupta, S. C., Pandey, M. K., Tyagi, A. K., & Deb, L. (2016). Oxidative stress and Cancer: Advances and challenges. Oxidative Medicine and Cellular Longevity, 2016, 1–1. https://doi.org/10.1155/2016/5010423.
Acknowledgements
We thank the Brazilian Board of Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher-Level-Education Personnel (CAPES) and the Foundation for Support to Technological Research and Innovation of the State of Sergipe (FAPITEC-SE) for the financial support.
Funding
Brazilian Board of Scientific and Technological Development (CNPq), the Coordination for the Improvement of Higher-Level-Education Personnel (CAPES) and the Foundation for Support to Technological Research and Innovation of the State of Sergipe (FAPITEC-SE).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Handling editor: Y. Robert Li
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations
Rights and permissions
About this article
Cite this article
Feitosa, L.A.d., Carvalho, J.d.S., Dantas, C.O. et al. Resistance training improves cardiac function and cardiovascular autonomic control in doxorubicin-induced cardiotoxicity. Cardiovasc Toxicol 21, 365–374 (2021). https://doi.org/10.1007/s12012-020-09627-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-020-09627-w