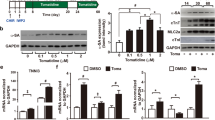
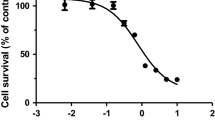
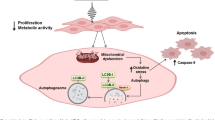
Abstract
H9c2 cells are used as a surrogate for cardiac cells in several toxicological studies, which are usually performed with cells in their undifferentiated state, raising questions on the applicability of the results to adult cardiomyocytes. Since H9c2 myoblasts have the capacity to differentiate into skeletal and cardiac muscle cells under different conditions, the hypothesis of the present work was that cells in different differentiation states differ in their susceptibility to toxicants. In order to test the hypothesis, the effects of the cardiotoxicant isoproterenol (ISO) were investigated. The present work demonstrates that differentiated H9c2 cells are more susceptible to ISO toxicity. Cellular content of beta1-adrenergic receptors (AR), beta3-AR, and calcineurin is decreased as cells differentiate, as opposed to the content on the mitochondrial voltage-dependent anion channel (VDAC) and phosphorylated p38-MAPK, which increase. After ISO treatment, the pro-apoptotic protein Bax increases in all experimental groups, although only undifferentiated myoblasts up-regulate the anti-apoptotic Bcl-2. Calcineurin is decreased in differentiated H9c2 cells, which suggests an important role against ISO-induced cell death. The results indicate that the differentiation state of H9c2 myoblasts influence ISO toxicity, which may involve calcineurin, p38-MAPK, and Bax/Bcl-2 alterations. The data also provide new insights into cardiovascular toxicology during early development.





Similar content being viewed by others
References
Limana, F., Zacheo, A., Mocini, D., Mangoni, A., Borsellino, G., Diamantini, A., et al. (2007). Identification of myocardial and vascular precursor cells in human and mouse epicardium. Circulation Research, 101, 1255–1265.
Hescheler, J., Meyer, R., Plant, S., Krautwurst, D., Rosenthal, W., & Schultz, G. (1991). Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circulation Research, 69, 1476–1486.
Kimes, B. W., & Brandt, B. L. (1976). Properties of a clonal muscle cell line from rat heart. Experimental Cell Research, 98, 367–381.
Schopf, G., Rumpold, H., & Muller, M. M. (1986). Alterations of purine salvage pathways during differentiation of rat heart myoblasts towards myocytes. Biochimica et Biophysica Acta, 884, 319–325.
Hammes, A., Oberdorf, S., Strehler, E. E., Stauffer, T., Carafoli, E., Vetter, H., et al. (1994). Differentiation-specific isoform mRNA expression of the calmodulin-dependent plasma membrane Ca(2+)-ATPase. FASEB Journal, 8, 428–435.
Hunter, A. L., Zhang, J., Chen, S. C., Si, X., Wong, B., Ekhterae, D., et al. (2007). Apoptosis repressor with caspase recruitment domain (ARC) inhibits myogenic differentiation. FEBS Letter, 581, 879–884.
Bregant, E., Renzone, G., Lonigro, R., Passon, N., Di Loreto, C., Pandolfi, M., et al. (2009). Down-regulation of SM22/transgelin gene expression during H9c2 cells differentiation. Molecular and Cellular Biochemistry, 327, 145–152.
Menard, C., Pupier, S., Mornet, D., Kitzmann, M., Nargeot, J., & Lory, P. (1999). Modulation of L-type calcium channel expression during retinoic acid-induced differentiation of H9C2 cardiac cells. Journal of Biological Chemistry, 274, 29063–29070.
Sardao, V. A., Oliveira, P. J., Holy, J., Oliveira, C. R., & Wallace, K. B. (2007). Vital imaging of H9c2 myoblasts exposed to tert-butylhydroperoxide—Characterization of morphological features of cell death. BMC Cell Biology, 8, 11.
Sardao, V. A., Oliveira, P. J., Holy, J., Oliveira, C. R., & Wallace, K. B. (2009). Doxorubicin-induced mitochondrial dysfunction is secondary to nuclear p53 activation in H9c2 cardiomyoblasts. Cancer Chemotherapy and Pharmacology, 64, 811–827.
Sardao, V. A., Oliveira, P. J., Holy, J., Oliveira, C. R., & Wallace, K. B. (2009). Morphological alterations induced by doxorubicin on H9c2 myoblasts: Nuclear, mitochondrial, and cytoskeletal targets. Cell Biology and Toxicology, 25, 227–243.
Lund, K. C., & Wallace, K. B. (2004). Direct effects of nucleoside reverse transcriptase inhibitors on rat cardiac mitochondrial bioenergetics. Mitochondrion, 4, 193–202.
Saito, S., Hiroi, Y., Zou, Y., Aikawa, R., Toko, H., Shibasaki, F., et al. (2000). beta-Adrenergic pathway induces apoptosis through calcineurin activation in cardiac myocytes. Journal of Biological Chemistry, 275, 34528–34533.
Shizukuda, Y., & Buttrick, P. M. (2002). Subtype specific roles of beta-adrenergic receptors in apoptosis of adult rat ventricular myocytes. Journal of Molecular and Cellular Cardiology, 34, 823–831.
Dangel, V., Giray, J., Ratge, D., & Wisser, H. (1996). Regulation of beta-adrenoceptor density and mRNA levels in the rat heart cell-line H9c2. Biochemical Journal, 317(Pt 3), 925–931.
Colucci, W. S., Sawyer, D. B., Singh, K., & Communal, C. (2000). Adrenergic overload and apoptosis in heart failure: Implications for therapy. Journal of Cardiac Failure, 6, 1–7.
Singh, K., Communal, C., Sawyer, D. B., & Colucci, W. S. (2000). Adrenergic regulation of myocardial apoptosis. Cardiovascular Research, 45, 713–719.
Goldspink, D. F., Burniston, J. G., & Tan, L. B. (2003). Cardiomyocyte death and the ageing and failing heart. Experimental Physiology, 88, 447–458.
Houghton, P., Fang, R., Techatanawat, I., Steventon, G., Hylands, P. J., & Lee, C. C. (2007). The sulphorhodamine (SRB) assay and other approaches to testing plant extracts and derived compounds for activities related to reputed anticancer activity. Methods, 42, 377–387.
Pereira, G. C., Branco, A. F., Matos, J. A., Pereira, S. L., Parke, D., Perkins, E. L., et al. (2007). Mitochondrially targeted effects of berberine [Natural Yellow 18,5,6-dihydro-9, 10-dimethoxybenzo(g)-1,3-benzodioxolo(5, 6-a) quinolizinium] on K1735–M2 mouse melanoma cells: comparison with direct effects on isolated mitochondrial fractions. Journal of Pharmacology and Experimental Therapeutics, 323, 636–649.
Serafim, T. L., Oliveira, P. J., Sardao, V. A., Perkins, E., Parke, D., & Holy, J. (2008). Different concentrations of berberine result in distinct cellular localization patterns and cell cycle effects in a melanoma cell line. Cancer Chemotherapy and Pharmacology, 61, 1007–1018.
Kageyama, K., Ihara, Y., Goto, S., Urata, Y., Toda, G., Yano, K., et al. (2002). Overexpression of calreticulin modulates protein kinase B/Akt signaling to promote apoptosis during cardiac differentiation of cardiomyoblast H9c2 cells. Journal of Biological Chemistry, 277, 19255–19264.
Brostrom, M. A., Reilly, B. A., Wilson, F. J., & Brostrom, C. O. (2000). Vasopressin-induced hypertrophy in H9c2 heart-derived myocytes. International Journal of Biochemistry and Cell Biology, 32, 993–1006.
Hou, Q., & Hsu, Y. T. (2005). Bax translocates from cytosol to mitochondria in cardiac cells during apoptosis: development of a GFP-Bax-stable H9c2 cell line for apoptosis analysis. American Journal of Physiology. Heart and Circulatory Physiology, 289, H477–H487.
Kim, J. Y., Lee, J. J., & Kim, K. S. (2003). Acetyl-CoA carboxylase beta expression mediated by MyoD and muscle regulatory factor 4 is differentially affected by retinoic acid receptor and retinoid X receptor. Exp Mol Med, 35, 23–29.
Kuzmenkin, A., Liang, H., Xu, G., Pfannkuche, K., Eichhorn, H., Fatima, A., et al. (2009). Functional characterization of cardiomyocytes derived from murine induced pluripotent stem cells in vitro. FASEB Journal, 23, 4168–4180.
Decker, R. S., Rines, A. K., Nakamura, S., Naik, T. J., Wassertsrom, J. A., & Ardehali, H. (2009). Phosphorylation of contractile proteins in response to alpha- and beta-adrenergic stimulation in neonatal cardiomyocytes. Translational Research, 155, 27–34.
Zhou, B., Wu, L. J., Tashiro, S., Onodera, S., Uchiumi, F., & Ikejima, T. (2007). Activation of extracellular signal-regulated kinase during silibinin-protected, isoproterenol-induced apoptosis in rat cardiac myocytes is tyrosine kinase pathway-mediated and protein kinase C-dependent. Acta Pharmacologica Sinica, 28, 803–810.
Shoshan-Barmatz, V., Keinan, N., & Zaid, H. (2008). Uncovering the role of VDAC in the regulation of cell life and death. Journal of Bioenergetics and Biomembranes, 40, 183–191.
Green, P. S., & Leeuwenburgh, C. (2002). Mitochondrial dysfunction is an early indicator of doxorubicin-induced apoptosis. Biochimica et Biophysica Acta, 1588, 94–101.
Spallarossa, P., Garibaldi, S., Altieri, P., Fabbi, P., Manca, V., Nasti, S., et al. (2004). Carvedilol prevents doxorubicin-induced free radical release and apoptosis in cardiomyocytes in vitro. Journal of Molecular and Cellular Cardiology, 37, 837–846.
Yu, X. Y., Geng, Y. J., Liang, J. L., Lin, Q. X., Lin, S. G., Zhang, S., et al. (2010). High levels of glucose induce apoptosis in cardiomyocyte via epigenetic regulation of the insulin-like growth factor receptor. Experimental Cell Research, 316, 2903–2909.
Watkins, S. J., Borthwick, G. M., & Arthur, H. M. (2010). The H9C2 cell line and primary neonatal cardiomyocyte cells show similar hypertrophic responses in vitro. In vitro cellular & developmental biology. Animal, 47(2), 125–131.
De Angelis, A., Piegari, E., Cappetta, D., Marino, L., Filippelli, A., Berrino, L. et al. (2009). Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation 121, 276–292.
Remiao, F., Carmo, H., Carvalho, F., & Bastos, M. L. (2001). Copper enhances isoproterenol toxicity in isolated rat cardiomyocytes: Effects on oxidative stress. Cardiovascular Toxicology, 1, 195–204.
Brodde, O. E. (1988). The functional importance of beta 1 and beta 2 adrenoceptors in the human heart. American Journal of Cardiology, 62, 24C–29C.
Communal, C., Singh, K., Sawyer, D. B., & Colucci, W. S. (1999). Opposing effects of beta(1)- and beta(2)-adrenergic receptors on cardiac myocyte apoptosis : Role of a pertussis toxin-sensitive G protein. Circulation, 100, 2210–2212.
Li, J., Yan, B., Huo, Z., Liu, Y., Xu, J., Sun, Y., et al. (2010). Beta2- but not beta1-adrenoceptor activation modulates intracellular oxygen availability. Journal of Physiology, 588, 2987–2998.
El-Armouche, A., & Eschenhagen, T. (2009). Beta-adrenergic stimulation and myocardial function in the failing heart. Heart Failure Reviews, 14, 225–241.
Dessy, C., & Balligand, J. L. (2010). Beta3-adrenergic receptors in cardiac and vascular tissues emerging concepts and therapeutic perspectives. Advances in Pharmacology, 59, 135–163.
York, M., Scudamore, C., Brady, S., Chen, C., Wilson, S., Curtis, M., et al. (2007). Characterization of troponin responses in isoproterenol-induced cardiac injury in the Hanover Wistar rat. Toxicologic Pathology, 35, 606–617.
Del Carlo, C. H., Pereira-Barretto, A. C., Cassaro-Strunz, C. M., Latorre Mdo, R., Oliveira Junior, M. T., & Ramires, J. A. (2009). Cardiac troponin T for risk stratification in decompensated chronic heart failure. Arquivos brasileiros de cardiologia, 92(5), 372–380, 389–397, 404–412.
Halestrap, A. P., McStay, G. P., & Clarke, S. J. (2002). The permeability transition pore complex: Another view. Biochimie, 84, 153–166.
Jacobson, J., & Duchen, M. R. (2002). Mitochondrial oxidative stress and cell death in astrocytes—requirement for stored Ca2+ and sustained opening of the permeability transition pore. Journal of Cell Science, 115, 1175–1188.
Sharaf El Dein, O., Gallerne, C., Deniaud, A., Brenner, C., & Lemaire, C. (2009). Role of the permeability transition pore complex in lethal inter-organelle crosstalk. Front Biosci, 14, 3465–3482.
Biary, N., & Akar, F. G. (2010). A brighter side of ROS revealed by selective activation of beta-adrenergic receptor subtypes. Journal of Physiology, 588, 2973–2974.
Rong, Y., & Distelhorst, C. W. (2008). Bcl-2 protein family members: Versatile regulators of calcium signaling in cell survival and apoptosis. Annual Review of Physiology, 70, 73–91.
Fiedler, B., & Wollert, K. C. (2005). Targeting calcineurin and associated pathways in cardiac hypertrophy and failure. Expert Opinion on Therapeutic Targets, 9, 963–973.
Ullrich, V., Namgaladze, D., & Frein, D. (2003). Superoxide as inhibitor of calcineurin and mediator of redox regulation. Toxicology Letters, 139, 107–110.
Sayen, M. R., Gustafsson, A. B., Sussman, M. A., Molkentin, J. D., & Gottlieb, R. A. (2003). Calcineurin transgenic mice have mitochondrial dysfunction and elevated superoxide production. American Journal of Physiology. Cell Physiology, 284, C562–C570.
Aramburu, J., Heitman, J., & Crabtree, G. R. (2004). Calcineurin: A central controller of signalling in eukaryotes. EMBO Report, 5, 343–348.
De Windt, L. J., Lim, H. W., Taigen, T., Wencker, D., Condorelli, G., Dorn, G. W., 2nd, et al. (2000). Calcineurin-mediated hypertrophy protects cardiomyocytes from apoptosis in vitro and in vivo: An apoptosis-independent model of dilated heart failure. Circulation Research, 86, 255–263.
Lim, M. J., Seo, Y. H., Choi, K. J., Cho, C. H., Kim, B. S., Kim, Y. H., et al. (2007). Suppression of c-Src activity stimulates muscle differentiation via p38 MAPK activation. Archives of Biochemistry and Biophysics, 465, 197–208.
Zetser, A., Gredinger, E., & Bengal, E. (1999). p38 mitogen-activated protein kinase pathway promotes skeletal muscle differentiation. Participation of the Mef2c transcription factor. Journal of Biological Chemistry, 274, 5193–5200.
Wu, Z., Woodring, P. J., Bhakta, K. S., Tamura, K., Wen, F., Feramisco, J. R., et al. (2000). p38 and extracellular signal-regulated kinases regulate the myogenic program at multiple steps. Molecular and Cellular Biology, 20, 3951–3964.
Tsang, M. Y., & Rabkin, S. W. (2009). p38 mitogen-activated protein kinase (MAPK) is activated by noradrenaline and serves a cardioprotective role, whereas adrenaline induces p38 MAPK dephosphorylation. Clinical and Experimental Pharmacology and Physiology, 36, e12–e19.
Clements, P., Brady, S., York, M., Berridge, B., Mikaelian, I., Nicklaus, R., et al. (2010). Time course characterization of serum cardiac troponins, heart fatty acid-binding protein, and morphologic findings with isoproterenol-induced myocardial injury in the rat. Toxicologic Pathology, 38, 703–714.
Feng, W., & Li, W. (2010). The study of ISO induced heart failure rat model. Experimental and Molecular Pathology, 88, 299–304.
Heather, L. C., Catchpole, A. F., Stuckey, D. J., Cole, M. A., Carr, C. A., & Clarke, K. (2009). Isoproterenol induces in vivo functional and metabolic abnormalities: similar to those found in the infarcted rat heart. Journal of Physiology and Pharmacology, 60, 31–39.
Slotkin, T. A., Lappi, S. E., & Seidler, F. J. (1995). Beta-adrenergic control of c-fos expression in fetal and neonatal rat tissues: relationship to cell differentiation and teratogenesis. Toxicology and Applied Pharmacology, 133, 188–195.
Iwasaki, T., Takino, Y., & Suzuki, T. (1990). Effects of isoproterenol on the developing heart in rats. Japanese Circulation Journal, 54, 109–116.
Acknowledgments
We are very grateful to Dr. Kendall B. Wallace (University of Minnesota, Medical School, Duluth, USA) for useful discussions and for providing initial funding for this study. This work was supported by a research grant from the Portuguese Foundation for Science and Technology (FCT), reference PTDC/QUI/64358/2006, to Paulo J. Oliveira. Ana Branco, Carolina Moreira, Sandro Pereira, and Vilma Sardão are supported by fellowships from the FCT (SFRH/BD/41384/2007, SFRH/BD/33892/2009, SFRH/BD/37933/2007, and SFRH/BPD/31549/2006, respectively).
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article can be found at http://dx.doi.org/10.1007/s12012-011-9131-1
Rights and permissions
About this article
Cite this article
Branco, A.F., Pereira, S.L., Moreira, A.C. et al. Isoproterenol Cytotoxicity is Dependent on the Differentiation State of the Cardiomyoblast H9c2 Cell Line. Cardiovasc Toxicol 11, 191–203 (2011). https://doi.org/10.1007/s12012-011-9111-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-011-9111-5