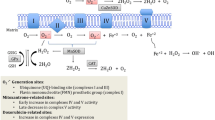
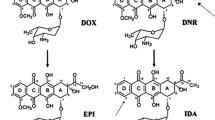
Abstract
Antitumor therapy with doxorubicin and other anthracyclines is limited by the possible development of cardiomyopathy upon chronic administration. Several lines of evidence suggest that a close link exists between cardiotoxicity and the amount of anthracycline that accumulates in the heart and then undergoes one- or two- electron reduction to toxic metabolites or by-products. Alternative metabolic pathways lead to an oxidative degradation of anthracyclines, possibly counteracting anthracycline accumulation and reductive bioactivation; unfortunately, however, the actual role of anthracycline oxidation is only partially characterized. Here, we briefly review the biochemical foundations of reductive versus oxidative anthracycline metabolism. We show that multiple links exist between one pathway of toxic biactivation and another, limiting the search and clinical development of “better anthracyclines” that retain antitumor activity but induce less cardiotoxicity than the available analogues.



Similar content being viewed by others
References
Meissner, K., Sperker, B., Karsten, C, Zu Schwabedissen, H. M., Seeland, U., Bohm, M., Bien, S., Dazert, P., Kunert-Keil, C., Vogelgesang, S., Warzok, R., Siegmund, W., Cascorbi, I., Wendt, M., & Kroemer, H. K. (2002). Expression and localization of P-glycoprotein in human heart: Effects of cardiomyopathy. The Journal of Histochemistry and Cytochemistry, 50, 1351–1356.
Minotti, G., Menna, P., Salvatorelli, E., Cairo, G., & Gianni, L. (2004). Anthracyclines: Molecular advances and pharmacologic developments in antitumor activity and cardiotoxicity. Pharmacological Reviews, 56, 185–229.
Cusack, B. J., Young, S. P., Driskell, J., & Olson, R. D. (1993). Doxorubicin and doxorubicinol pharmacokinetics and tissue concentrations following bolus injection and continuous infusion of doxorubicin in the rabbit. Cancer Chemotherapy and Pharmacology, 32, 53–58.
Minotti, G., Recalcati, S., Menna, P., Salvatorelli, E., Corna, G., & Cairo, G. (2004). Doxorubicin cardiotoxicity and the control of iron metabolism: Quinone dependent and independent mechanisms. Methods in Enzymology, 378, 340–361.
Menna, P., Salvatorelli, E., Giampietro, R., Liberi, G., Teodori, G., Calafiore, A. M., & Minotti, G. (2002). Doxorubicin-dependent reduction of ferrylmyoglobin and inhibition of lipid peroxidation: Implications for cardiotoxicity of anticancer anthracyclines. Chemical Research in Toxicology, 15, 1179–1189.
Cartoni, A., Menna, P., Salvatorelli, E., Braghiroli, D., Giampietro, R., Animati, F., Urbani, A., Del Boccio, P., & Minotti, G. (2004). Oxidative degradation of cardiotoxic anticancer anthracyclines to phthalic acids : Novel function for ferrylmyoglobin? The Journal of Biological Chemistry, 279, 5088–5099.
Cairo, G., & Pietrangelo, A. (2000). Iron regulatory proteins in pathobiology. The Biochemical Journal, 352, 241–250.
Galy, B., Ferring, D., Benesova, M., Benes, V., & Hentze, M. W. (2004). Targeted mutagenesis of the murine IRP1 and IRP2 genes reveals context-dependent RNA processing differences in vivo. RNA, 10, 1019–1025.
Iwai, K., Drake, S. K., Wehr, N. B., Weissman, A. M., LaVaute, T., Minato, N., Klausner, R. D., Levine, R. L., & Rouault, T. A. (1998). Iron-dependent oxidation, ubiquitination, and degradation of iron regulatory protein 2: Implications for degradation of oxidized proteins. Proceedings of the National Academy of Sciences of the United States of America, 95, 4924–4928.
Minotti, G., Ronchi, R., Salvatorelli, E., Menna, P., & Cairo, G. (2001). Doxorubicin irreversibly inactivates Iron Regulatory Proteins 1 and 2 in cardiomyocytes : Evidence for distinct metabolic pathways and implications for iron-mediated cardiotoxicity of antitumor therapy. Cancer Research, 61, 8422–8428.
Corna, G., Santambrogio, P., Minotti, G., & Cairo, G. (2004). Doxorubicin paradoxically protects cardiomyocytes against iron-mediated toxicity: Role of reactive oxygen species and ferritin. The Journal of Biological Chemistry, 279, 13738–13745.
Cairo, G., Recalcati, S., Pietrangelo, A., & Minotti, G. (2002). The iron regulatory proteins: Targets of free radicals and modulators of oxidative damage. Free Radical Biology & Medicine, 32, 1237–1243.
Cairo, G., Ronchi, R., Recalcati, S., & Minotti, G. (2003). Nitric oxide and peroxynitrite activate the Iron Regulatory Protein-1 of J774A.1 macrophages by direct disassembly of the Fe-S cluster of cytoplasmic aconitase. Biochemistry, 41, 7435–7442.
Gewirtz, D. A. (1999). A critical evaluation of the mechanisms of action proposed for the antitumor effects of the anthracycline antibiotics adriamycin and daunorubicin. Biochemical Pharmacology, 57, 727–741.
Minotti, G., Recalcati, S., Mordente, A., Liberi, G., Calafiore, A. M., Mancuso, C., Preziosi, P., & Cairo, G. (1998). The secondary alcohol metabolite of doxorubicin irreversibly inactivates aconitase/iron regulatory protein-1 in cytosolic fractions from human myocardium. FASEB Journals, 12, 541–552.
Kwok, J. C., & Richardson, D. R. (2002). Unexpected anthracycline-mediated alterations in iron-regulatory protein-RNA-binding activity: The iron and copper complexes of anthracyclines decrease RNA-binding activity. Molecular Pharmacology, 62, 888–900.
Kwok, J. C., & Richardson, D. R. (2004). Examination of the mechanism(s) involved in doxorubicin-mediated iron accumulation in ferritin: studies using metabolic inhibitors, protein synthesis inhibitors, and lysosomotropic agents. Molecular Pharmacology, 65, 181–195.
Cairo, G., Bernuzzi, F., & Recalcati, S. (2006). A precious metal: Iron, an essential nutrient for all cells. Genes and Nutrition, 1, 25–40.
Cusack, B. J., Gambliel, H., Musser, B., Hadjokas, N., Shadle, S. E., Charlier, H., & Olson, R. D. (2006). Prevention of chronic anthracycline cardiotoxicity in the adult Fischer 344 rat by dexrazoxane and effects on iron metabolism. Cancer Chemotherapy and Pharmacology, 58, 517–526.
Sacco, G., Giampietro, R., Salvatorelli, E., Menna, P., Bertani, N., Graiani, G., Animati, F., Goso, C., Maggi, C. A., Manzini, S., & Minotti, G. (2003). Chronic cardiotoxicity of anticancer anthracyclines in the rat: Role of secondary alcohol metabolites and reduced toxicity by a novel anthracycline with impaired metabolite formation and reactivity. British Journal of Pharmacology, 139, 641–651.
Corna, R., Galy, B., & Cairo, G. (2006). IRP1-independent alterations of cardiac iron metabolism in doxorubicin-treated mice. Journal of Molecular Medicine, 84, 551–560.
Minotti, G., Cairo, G., & Monti, E. (1999). Role of iron in anthracycline cardiotoxicity: New tunes for an old song? FASEB Journals, 13, 199–212.
Galy, B., Ferring, D., & Hentze, M. W. (2005). Generation of conditional alleles of the murine Iron Regulatory Protein (IRP)-1 and -2 genes. Genesis, 43, 181–188.
Ladas, E. J., Jacobson, J. S., Kennedy, D. D., Teel, K., Fleischauer, A., & Kelly, K. M. (2004). Antioxidants and cancer therapy: a systematic review. Journal of Clinical Oncology, 22, 517–528.
Sokolove, P. M. (1994). Interactions of adriamycin aglycones with mitochondria may mediate adriamycin cardiotoxicity. International Journal of Biochemistry, 26, 1341–1350.
Gille, L., & Nohl, H. (1997). Analyses of the molecular mechanism of adriamycin-induced cardiotoxicity. Free Radical Biology & Medicine, 23, 775–782.
Olson, R. D., & Mushlin, P. S. (1990). Doxorubicin cardiotoxicity: Analysis of prevailing hypotheses. FASEB Journals, 4, 3076–3086.
Olson, R. D., Li, X., Palade, P., Shadle, S. E., Mushlin, P. S., Gambliel, H. A., Fill, M., Boucek, R. J. Jr, & Cusack, B. J. (2000). Sarcoplasmic reticulum calcium release is stimulated and inhibited by daunorubicin and daunorubicinol. Toxicology and Applied Pharmacology, 169, 168–176.
Gambliel, H. A., Burke, B. E., Cusack, B. J., Walsh, G. M., Zhang, Y. L., Mushlin, P. S., & Olson, R. D. (2002). Doxorubicin and C-13 deoxydoxorubicin effects on ryanodine receptor gene expression. Biochemical and Biophysical Research Communications, 291, 433–438.
Burke, B. E., Gambliel, H., Olson, R. D., Bauer, F. K., & Cusack, B. J. (2000). Prevention by dexrazoxane of down-regulation of ryanodine receptor gene expression in anthracycline cardiomyopathy in the rat. British Journal of Pharmacology, 131, 1–4.
Menna, P., Minotti, G., & Salvatorelli, E. (2007). In vitro modelling of the structure-activity determinants of anthracycline cardiotoxicity. Cell Biology and Toxicology, 23, 49–62.
Fiedler, W., Tchen, N., Bloch, J., Fargeot, P., Sorio, R., Vermorken, J. B., Collette, L., Lacombe, D., & Twelves, C. (2006). A study from the EORTC new drug development group: Open label phase II study of sabarubicin (MEN-10755) in patients with progressive hormone refractory prostate cancer. European Journal of Cancer, 42, 200–204.
Salvatorelli, E., Guarnieri, S., Menna, P., Liberi, G., Calafiore, A., Mariggio’, M. A., Mordente, A., Gianni, L., & Minotti, G. (2006). Defective one or two electron reduction of the anticancer anthracycline epirubicin in human heart: Relative importance of vesicular sequestration and impaired efficiency of electron addition. The Journal of Biological Chemistry, 281, 10990–11001.
Acknowledgments
This work was supported by Associazione Italiana Ricerca sul Cancro and Ministero dell’ Universita’ e Ricerca Scientifica e Tecnologica (MIUR) (Center of Excellence on Aging at the University of Chieti).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Menna, P., Recalcati, S., Cairo, G. et al. An introduction to the metabolic determinants of anthracycline cardiotoxicity. Cardiovasc Toxicol 7, 80–85 (2007). https://doi.org/10.1007/s12012-007-0011-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12012-007-0011-7