Abstract
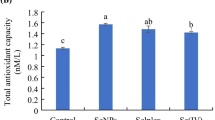
A study was conducted to determine the effect of dietary selenium (Se) concentration and source for broiler chickens on performance, nutrient digestibility, plasma Se, glutathione peroxidase (GPx) activity, and thiobarbituric acid reactive substances (TBARS). A total of 700 1-day-old broiler chicks were assigned to 7 diets with 20 birds per cage and 5 replicates per treatment. The experimental diets were fed for 32 days in 2 phases (phase 1, day 0 to 14 and phase 2, day 15 to 32). Treatments were as follows: control (without Se supplementation), sodium selenite (SeS; 0.15, 0.30, or 0.45 ppm), and hot-melt extruded sodium selenite (SeHME; 0.15, 0.30, or 0.45 ppm). There were significant linear responses (P < 0.01) for higher plasma Se concentration in SeS and SeHME treatments. Moreover, an increased (P < 0.01) Se concentration of plasma occurred in SeHME treatment compared with that in SeS treatment. The serum GPx analyses revealed that supplemental SeS and SeHME increased significantly the activity of GPx in the plasma in phase 1 (P < 0.05) and phase 2 (P < 0.05). There were significant linear (P < 0.01) responses of SeS and SeHME treatments for the expression of SelW, GPx1, GPx3, and GPx4 in the livers and spleens. In addition, SeHME showed an upregulated expression of GPx-4 in the livers (P < 0.01) and SelW in the spleens (P < 0.05) compared with SeS treatment. SeHME showed a lower TBARS on day 9. Moreover, a decreased (P < 0.01) TBARS occurred in SeS treatment compared with that in control treatment. In conclusion, SeHME can increase antioxidant activity and Se absorption, consequently being a more suitable source of Se than regular sodium selenite.
Similar content being viewed by others
References
Diplock AT (1992) Selenium, antioxidant nutritions, and human diseases. Biol Trace Elem Res 33:155–156. https://doi.org/10.1007/BF02784005
Suchy P, Strakova E, Herzig I (2014) Selenium in poultry nutrition: a review. Czech J Anim Sci 59:495–503. https://doi.org/10.17221/7730-CJAS
Skalickova S, Milosavljevic V, Cihalova K, Horky P, Richtera L, Adam V (2017) Selenium nanoparticles as a nutritional supplement. Nutr 33:83–90. https://doi.org/10.1016/j.nut.2016.05.001
Payne RL, Southern LL (2005) Comparison of inorganic and organic selenium sources for broilers. Poult Sci 84:898–902. https://doi.org/10.1093/ps/84.6.898
Hu CH, Li YL, Xiong L, Zhang HM, Song J, Xia MS (2012) Comparative effects of nano elemental selenium and sodium selenite on selenium retention in broiler chickens. Anim Feed Sci Technol 177:204–210. https://doi.org/10.1016/j.anifeedsci.2012.08.010
Thiry C, Schneider YJ, Pussemier L, De Temmerman L, Ruttens A (2013) Selenium bioaccessibility and bioavailability in Se-enriched food supplements. Biol Trace Elem Res 152:152–160. https://doi.org/10.1007/s12011-013-9604-0
Boostani A, Sadeghi AA, Mousavi SN, Chamani M, Kashan N (2015) Effects of organic, inorganic, and nano-Se on growth performance, antioxidant capacity, cellular and humoral immune responses in broiler chickens exposed to oxidative stress. Livest Sci 178:330–336. https://doi.org/10.1016/j.livsci.2015.05.004
Mohammadi V, Ghazanfari S, Mohammadi-Sangcheshmeh A, Nazaran MH (2015) Comparative effects of zinc-nano complexes, zinc-sulphate and zinc-methionine on performance in broiler chickens. Br Poult Sci 56:486–493. https://doi.org/10.1080/00071668.2015.1064093
Zhou X, Wang Y (2011) Influence of dietary nano elemental selenium on growth performance, tissue selenium distribution, meat quality, and glutathione peroxidase activity in Guangxi Yellow chicken. Poult Sci 90:680–686. https://doi.org/10.3382/ps.2010-00977
Ahmadi M, Ahmadian A, Seidavi AR (2018) Effect of different levels of nano-selenium on performance, blood parameters, immunity and carcass characteristics of broiler chickens. Poult Sci 6:9–108. https://doi.org/10.22069/PSJ.2018.13815.1276
Lin X, Yang T, Li H, Ji Y, Zhao Y, He J (2019) Interactions between different selenium compounds and essential trace elements involved in the antioxidant system of laying hens. Biol Trace Elem Res (Online published. https://doi.org/10.1007/s12011-019-01701-x
Wang Y (2009) Differential effects of sodium selenite and nano-Se on growth performance, tissue Se distribution, and glutathione peroxidase activity of avian broiler. Biol Trace Elem Res 128(2):184–190. https://doi.org/10.1007/s12011-008-8264-y
Lee SY, Nam S, Choi Y, Kim M, Koo JS, Chae BJ, Kang WS, Cho HJ (2017) Fabrication and characterizations of hot-melt extruded nanocomposites based on zinc sulfate monohydrate and soluplus. Appl Sci 7:902. https://doi.org/10.3390/app7090902
Aviagen (2014) Ross broiler management manual. http://pt.aviagen.com/assets/Tech_Center/Ross_Broiler/Ross_Broiler_Manual. 9:350–364
Jin X, Jia T, Liu R, Xu S (2018) The antagonistic effect of selenium on cadmium-induced apoptosis via PPAR-γ/PI3K/Akt pathway in chicken pancreas. J Hazard Mater 357:355–362. https://doi.org/10.1016/j.jhazmat.2018.06.003
Sinnhuber RO, Yu TC (1977) The 2-thiobarbituric acid reaction, an objective measure of the oxidative deterioration occurring in fats and oils. J Jap Soc Fish Sci 26:259–267. https://doi.org/10.5650/jos1956.26.259
Littell RC, Henry PR, Lewis AJ, Ammerman CB (1997) Estimation of relative bioavailability of nutrients using SAS procedures. J Anim Sci 75:2672–2683. https://doi.org/10.2527/1997.75102672x
Biller-Takahashi JD, Takahashi LS, Mingatto FE, Urbinati EC (2015) The immune system is limited by oxidative stress: dietary selenium promotes optimal antioxidative status and greatest immune defense in pacu Piaractus mesopotamicus. Fish Shellfish Immunol 47:360–367. https://doi.org/10.1016/j.fsi.2015.09.022
Pisoschi AM, Pop A (2015) The role of antioxidants in the chemistry of oxidative stress: a review. Eur J Med Chem 97:55–74. https://doi.org/10.1016/j.ejmech.2015.04.040
Bakhshalinejad R, Akbari Moghaddam Kakhki R, Zoidis E (2018) Effects of different dietary sources and levels of selenium supplements on growth performance, antioxidant status and immune parameters in Ross 308 broiler chickens. Br Poult Sci 59:81–91. https://doi.org/10.1080/00071668.2017.1380296
Gurkan F, Atamer Y, Ece A, Kocyigit Y, Tuzun H, Mete M (2004) Relationship among serum selenium levels, lipid peroxidation, and acute bronchiolitis in infancy. Biol Trace Elem Res 100:97–104. https://doi.org/10.1385/BTER:100:2:097
Sies H (1999) Glutathione and its role in cellular functions. Free Radic Biol Med 27:916–921. https://doi.org/10.1016/S0891-5849(99)00177-X
Markovic SD, Djačić DS, Cvetković DM, Obradović AD, Žižić JB, Ognjanović BI, Štajn AŠ (2011) Effects of acute in vivo cisplatin and selenium treatment on hematological and oxidative stress parameters in red blood cells of rats. Biol Trace Elem Res 142:660–670. https://doi.org/10.1007/s12011-010-8788-9
Pilarczyk B, Drozd R, Pilarczyk R, Tomza-Marciniak A, Jankowiak D, Hendzel D, Kuba J, Kowalska J (2011) Glutathione peroxidase (GSHPx) activity in the liver of red deer in relation to hepatic selenium concentrations, sex, body weight and season of the year. Biol Trace Elem Res 144:560–569. https://doi.org/10.1007/s12011-011-9022-0
Yao H, Zhao W, Zhao X, Fan R, Khoso PA, Zhang Z, Liu W, Xu S (2014) Selenium deficiency mainly influences the gene expressions of antioxidative selenoproteins in chicken muscles. Biol Trace Elem Res 161:318–327. https://doi.org/10.1007/s12011-014-0125-2
Reeves MA, Hoffmann PR (2009) The human selenoproteome: recent insights into functions and regulation. Cell Mol Life Sci 66:2457–2478. https://doi.org/10.1007/s00018-009-0032-4
Zia S, Islam F (2000) Selenium altered the levels of lipids, lipid peroxidation, and sulfhydryl groups in straitum and thalamus of rat. Biol Trace Elem Res 77:251–259. https://doi.org/10.1385/BTER:77:3:251
Chadio SE, Pappas AC, Papanastasatos A, Pantelia D, Dardamani A, Fegeros K, Zervas G (2015) Effects of high selenium and fat supplementation on growth performance and thyroid hormones concentration of broilers. J Trace Elem Med Biol 29:202–207. https://doi.org/10.1016/j.jtemb.2014.09.010
Habibian M, Sadeghi G, Ghazi S, Moeini MM (2015) Selenium as a feed supplement for heat-stressed poultry: a review. Biol Trace Elem Res 165:183–193. https://doi.org/10.1007/s12011-015-0317-4
Kachuee R, Abdi-Benemar H, Mansoori Y, Sánchez-Aparicio P, Seifdavati J, Elghandour MM, Guillén RJ, Salem AZ (2019) Effects of sodium selenite, L-selenomethionine, and selenium nanoparticles during late pregnancy on selenium, zinc, copper, and iron concentrations in Khalkhali goats and their kids. Biol Trace Elem Res 1–14. https://doi.org/10.1007/s12011-018-1618-1
Funding
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries (IPET) through Agri-Bio industry Technology Development Program, funded by Ministry of Agriculture, Food and Rural Affairs (MAFRA) (No.116073-3).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The protocol for the present experiment was approved by the Institutional Animal Care and Use Committee of Kangwon National University, Republic of Korea.
Conflict of Interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lee, J., Hosseindoust, A., Kim, M. et al. Biological Evaluation of Hot-Melt Extruded Nano-selenium and the Role of Selenium on the Expression Profiles of Selenium-Dependent Antioxidant Enzymes in Chickens. Biol Trace Elem Res 194, 536–544 (2020). https://doi.org/10.1007/s12011-019-01801-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-019-01801-8