Abstract
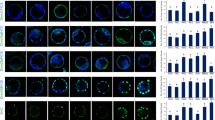
The aim of the present study was to investigate the effects of selenium (Se) deficiency on autophagy-related genes and on ultrastructural changes in the spleen, bursa of Fabricius, and thymus of chickens. The Se deficiency group was fed a basal diet containing Se at 0.033 mg/kg and the control group was fed the same basal diet containing Se at 0.15 mg/kg. The messenger RNA (mRNA) levels of the autophagy genes microtubule-associated protein 1 light chain 3 (LC3)-I, LC3-II, Beclin 1, dynein, autophagy associated gene 5 (ATG5), and target of rapamycin complex 1 (TORC1) were assessed using real-time qPCR. The protein levels of LC3-II, Beclin 1, and dynein were investigated using western blot analysis. Furthermore, the ultrastructure was observed using an electron microscope. The results indicated that spleen mRNA levels of LC3-I, LC3-II, Beclin 1, dynein, ATG5, and TORC1 and the protein levels of LC3-II, Beclin 1, and dynein were increased in the Se deficiency group compared with the control group. In the bursa of Fabricius, the mRNA levels of LC3-I, LC3-II, Beclin 1, dynein, ATG5, and TORC1 and the protein levels of Beclin 1 and dynein were increased; furthermore, the protein level of LC3-II was decreased in the Se deficiency group compared to the control group. In the thymus, the mRNA levels of LC3-I, Beclin 1, and ATG5 increased; the levels of LC3-II, dynein, and TORC1 were decreased; the protein level of Beclin 1 increased; and the levels of LC3-II and dynein decreased in the Se deficiency group compared to those in the control group. Further cellular morphological changes, such as autophagy vacuoles, autolysosomes, and lysosomal degradation, were observed in the spleen, bursa of Fabricius, and thymus of the Se-deficiency group. In summary, Se deficiency caused changes in autophagy-related genes, which increased the autophagic process and also caused structural damages to the immune organs of chickens.







Similar content being viewed by others
Abbreviations
- Se:
-
Selenium
- LC3:
-
Microtubule-associated protein1 light chain 3
- TOR:
-
Target of rapamycin
- TORC1:
-
Target of rapamycin complex l
- TORC2:
-
Target of rapamycin complex 2
- mTOR:
-
Mammalian target of rapamycin
- ATG:
-
Autophagy associated gene
- AVM:
-
Avermectin
References
Lei C, Niu XL, Ma XK, Wei J (2011) Is selenium deficiency really the cause of Keshan disease? Environ Geochem Hlth 33(2):183–188. doi:10.1007/s10653-010-9331-9
You L, Liu C, Yang ZJ, Li M, Li S (2014) Prediction of selenoprotein T structure and its response to selenium deficiency in chicken immune organs. Biol Trace Elem Res 160(2):222–231. doi:10.1007/s12011-014-0049-x
Willett WC, Polk BF, Morris JS, Stampfer MJ, Pressel S, Rosner B, Taylor JO, Schneider K, Hames CG (1983) Prediagnostic serum selenium and risk of cancer. Lancet 2(8342):130–134
Khoso PA, Yang Z, Liu C, Li S (2015) Selenium deficiency downregulates selenoproteins and suppresses immune function in chicken thymus. Biol Trace Elem Res 167(1):48–55. doi:10.1007/s12011-015-0282-y
Janda E, Isidoro C, Carresi C, Mollace V (2012) Defective autophagy in Parkinson’s disease: role of oxidative stress. Mol Neurobiol 46(3):639–661. doi:10.1007/s12035-012-8318-1
Zhang ZW, Wang QH, Zhang JL, Li S, Wang XL, Xu SW (2012) Effects of oxidative stress on immunosuppression induced by selenium deficiency in chickens. Biol Trace Elem Res 149(3):352–361. doi:10.1007/s12011-012-9439-0
Trincheri NF, Follo C, Nicotra G, Peracchio C, Castino R, Isidoro C (2008) Resveratrol-induced apoptosis depends on the lipid kinase activity of Vps34 and on the formation of autophagolysosomes. Carcinogenesis 29(2):381–389. doi:10.1093/carcin/bgm271
Levine B, Mizushima N, Virgin HW (2011) Autophagy in immunity and inflammation. Nature 469(7330):323–335. doi:10.1038/nature09782
Rong YG, McPhee CK, Deng SS, Huang L, Chen LL, Liu M, Tracy K, Baehrecke EH, Yu L, Lenardo MJ (2011) Spinster is required for autophagic lysosome reformation and mTOR reactivation following starvation (vol 108, pg 7826, 2011). Proc Natl Acad Sci U S A 108(27):11297–11297. doi:10.1073/pnas.1108410108
Xiong N, Xiong J, Jia M, Liu L, Zhang X, Chen Z, Huang J, Zhang Z, Hou L, Luo Z, Ghoorah D, Lin Z, Wang T (2013) The role of autophagy in Parkinson’s disease: rotenone-based modeling. Behav Brain Funct: BBF 9:13. doi:10.1186/1744-9081-9-13
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441(7095):880–884. doi:10.1038/nature04723
Yoshioka A, Miyata H, Doki Y, Yamasaki M, Sohma I, Gotoh K, Takiguchi S, Fujiwara Y, Uchiyama Y, Monden M (2008) LC3, an autophagosome marker, is highly expressed in gastrointestinal cancers. Int J Oncol 33(3):461–468. doi:10.3892/ijo_00000028
Kabeya Y, Mizushima N, Ueno T, Yamamoto A, Kirisako T, Noda T, Kominami E, Ohsumi Y, Yoshimori T (2003) LC3, a mammalian homolog of yeast Apg8p, is localized in autophagosome membranes after processing (vol 19, pg 5720, 2000). EMBO J 22(17):4577–4577
Lai YC, Hickey RW, Chen YM, Bayir H, Sullivan ML, Chu CT, Kochanek PM, Dixon CE, Jenkins LW, Graham SH, Watkins SC, Clark RSB (2008) Autophagy is increased after traumatic brain injury in mice and is partially inhibited by the antioxidant gamma-glutamylcysteinyl ethyl ester. J Cerebr Blood F Met 28(3):540–550. doi:10.1038/sj.jcbfm.9600551
Cui JZ, Gong ZY, Shen HM (2013) The role of autophagy in liver cancer: molecular mechanisms and potential therapeutic targets. Bba-Rev Cancer 1836(1):15–26. doi:10.1016/j.bbcan.2013.02.003
Mitchell DR (1994) Cell and molecular biology of flagellar dyneins. Int Rev Cytol 155:141–180
Noda T, Ohsumi Y (1998) Tor, a phosphatidylinositol kinase homologue, controls autophagy in yeast. J Biol Chem 273(7):3963–3966
Scott RC, Schuldiner O, Neufeld TP (2004) Role and regulation of starvation-induced autophagy in the Drosophila fat body. Dev Cell 7(2):167–178. doi:10.1016/j.devcel.2004.07.009
Zhang J, Chiu J, Zhang H, Qi T, Tang Q, Ma K, Lu H, Li G (2013) Autophagic cell death induced by resveratrol depends on the Ca(2+)/AMPK/mTOR pathway in A549 cells. Biochem Pharmacol 86(2):317–328. doi:10.1016/j.bcp.2013.05.003
JP Q, Li M, Zhao FQ, Liu C, Zhang ZW, SW X, Li S (2015) Autophagy is upregulated in brain tissues of pigeons exposed to avermectin. Ecotoxicol Environ Saf 113:159–168. doi:10.1016/j.ecoenv.2014.12.002
Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J (2007) Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res 100(6):914–922. doi:10.1161/01.RES.0000261924.76669.36
Virgin HW, Levine B (2009) Autophagy genes in immunity. Nat Immunol 10(5):461–470. doi:10.1038/ni.1726
Liu C, Li M, Cao Y, Qu JP, Zhang ZW, Xu SW, Li S (2014) Effects of avermectin on immune function and oxidative stress in the pigeon spleen. Chem Biol Interact 210:43–50. doi:10.1016/j.cbi.2013.12.015
Li JL, Li HX, Gao XJ, Zhang JL, Li S, SW X, Tang ZX (2012) Priority in selenium homeostasis involves regulation of SepSecS transcription in the chicken brain. PLoS One 7(4):e35761. doi:10.1371/journal.pone.0035761
Kiremidjian-Schumacher L, Roy M, Wishe HI, Cohen MW, Stotzky G (1992) Regulation of cellular immune responses by selenium. Biol Trace Elem Res 33:23–35
Demirci S, Kutluhan S, Naziroglu M, Uguz AC, Yurekli VA, Demirci K (2013) Effects of selenium and topiramate on cytosolic Ca(2+) influx and oxidative stress in neuronal PC12 cells. Neurochem Res 38(1):90–97. doi:10.1007/s11064-012-0893-z
Peng X, Cui H, Yuan J, Cui W, Fang J, Zuo Z, Deng J, Pan K, Zhou Y, Lai W (2011) Low-selenium diet induces cell cycle arrest of thymocytes and alters serum IL-2 content in chickens. Biol Trace Elem Res 144(1–3):688–694. doi:10.1007/s12011-011-9077-y
Khoso PA, Yang Z, Liu C, Li S (2015) Selenoproteins and heat shock proteins play important roles in immunosuppression in the bursa of Fabricius of chickens with selenium deficiency. Cell Stress Chaperones 20(6):967–978. doi:10.1007/s12192-015-0625-9
Wang CW, Klionsky DJ (2003) The molecular mechanism of autophagy. Mol Med 9(3–4):65–76
Shintani T, Klionsky DJ (2004) Autophagy in health and disease: a double-edged sword. Science 306(5698):990–995. doi:10.1126/science.1099993
Park MH, Lee SJ, Byun HR, Kim Y, YJ O, Koh JY, Hwang JJ (2011) Clioquinol induces autophagy in cultured astrocytes and neurons by acting as a zinc ionophore. Neurobiol Dis 42(3):242–251. doi:10.1016/j.nbd.2011.01.009
Mizushima N, Yoshimori T (2007) How to interpret LC3 immunoblotting. Autophagy 3(6):542–545
Zucchini-Pascal N, de Sousa G, Rahmani R (2009) Lindane and cell death: at the crossroads between apoptosis, necrosis and autophagy. Toxicology 256(1–2):32–41. doi:10.1016/j.tox.2008.11.004
He CC, Klionsky DJ (2009) Regulation mechanisms and signaling pathways of autophagy. Annu Rev Genet 43:67–93. doi:10.1146/annurev-genet-102808-114910
Ren HG, Fu K, CC M, Li B, Wang D, Wang GH (2010) DJ-1, a cancer and Parkinson’s disease associated protein, regulates autophagy through JNK pathway in cancer cells. Cancer Lett 297(1):101–108. doi:10.1016/j.canlet.2010.05.001
Gui YX, Fan XN, Wang HM, Wang G, Chen SD (2012) Glyphosate induced cell death through apoptotic and autophagic mechanisms. Neurotoxicol Teratol 34(3):342–349. doi:10.1016/j.ntt.2012.03.005
Maday S, Wallace KE, Holzbaur EL (2012) Autophagosomes initiate distally and mature during transport toward the cell soma in primary neurons. J Cell Biol 196(4):407–417. doi:10.1083/jcb.201106120
Winslow AR, Rubinsztein DC (2008) Autophagy in neurodegeneration and development. Biochim Biophys Acta 1782(12):723–729. doi:10.1016/j.bbadis.2008.06.010
Maskey D, Yousefi S, Schmid I, Zlobec I, Perren A, Friis R, Simon HU (2013) ATG5 is induced by DNA-damaging agents and promotes mitotic catastrophe independent of autophagy. Nat Commun 4. doi:10.1038/Ncomms3130
Pyo JO, Yoo SM, Ahn HH, Nah J, Hong SH, Kam TI, Jung S, Jung YK (2013) Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nat Commun 4:2300. doi:10.1038/ncomms3300
Chen B, Sun XJ, Zhang Y, Zhu XQ, Shen HM (2012) Use of inducible Atg5 deletion and expression cell lines in study of the pro-survival function of autophagy under starvation. Biochem Bioph Res Co 427(1):11–17. doi:10.1016/j.bbrc.2012.08.117
Liu C, Zhao Y, Chen L, Zhang Z, Li M, Li S (2015) Avermectin induced autophagy in pigeon spleen tissues. Chem Biol Interact 242:327–333. doi:10.1016/j.cbi.2015.10.022
Yao L, Du Q, Yao H, Chen X, Zhang Z, Xu S (2015) Roles of oxidative stress and endoplasmic reticulum stress in selenium deficiency-induced apoptosis in chicken liver. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine 28(2):255–265. doi:10.1007/s10534-014-9819-3
Sheng PF, Jiang Y, Zhang ZW, Zhang JL, Li S, Zhang ZQ, SW X (2014) The effect of Se-deficient diet on gene expression of inflammatory cytokines in chicken brain. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine 27(1):33–43. doi:10.1007/s10534-013-9682-7
Shao JJ, Yao HD, Zhang ZW, Li S, Xu SW (2012) The disruption of mitochondrial metabolism and ion homeostasis in chicken hearts exposed to manganese. Toxicol Lett 214(2):99–108. doi:10.1016/j.toxlet.2012.08.011
Li JL, Li HX, Li S, Gao XJ, SW X, Tang ZX (2012) Effects of selenoprotein W gene expression by selenium involves regulation of mRNA stability in chicken embryos neurons. Biometals : an international journal on the role of metal ions in biology, biochemistry, and medicine 25(2):459–468. doi:10.1007/s10534-012-9517-y
Acknowledgments
The authors are grateful to members of the Veterinary Physiology and Veterinary Internal Medicine Lab for tissue collection and processing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of Interests
The authors declare that they have no conflicts of interests.
Funding
This study was supported by the National Natural Science Foundation of China (Grant No. 31472161).
Humane Care of Animals
All of the chicken experiments were approved by the Institutional Animal Care and Use Committee of Northeast Agricultural University under the approved protocol number SRM-06.
Rights and permissions
About this article
Cite this article
Khoso, P.A., Pan, T., Wan, N. et al. Selenium Deficiency Induces Autophagy in Immune Organs of Chickens. Biol Trace Elem Res 177, 159–168 (2017). https://doi.org/10.1007/s12011-016-0860-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-016-0860-7