Abstract
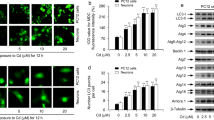
Cadmium (Cd) induces significant oxidative damage in cells. Recently, it was reported that autophagy could be induced by Cd in neurons. However, little is known about the role of reactive oxygen species (ROS) during Cd-induced autophagy. In our study, we examined the cross-talk between ROS and autophagy by using N-acetyl cysteine (NAC, an antioxidant) and chloroquine (CQ, a pharmacological inhibitor of autophagy) in a primary rat neuronal cell cultures. We observed accumulation of acidic vesicular organelles and the increased expression of endogenous protein light chain 3 (LC3) in Cd-treated neurons, revealing that Cd induced a high level of autophagy. Moreover, increased levels of ROS were observed in neurons treated with Cd, showing that ROS accumulation was closely associated with neuron’s exposure to Cd. Furthermore, we found that autophagy was inhibited by using CQ and/or NAC with further aggravation of mitochondrial damage, lactate dehydrogenase (LDH) leakage and hypoploid apoptotic cell number in Cd-treated neurons. These results proved that autophagy has a cytoprotective role during Cd-induced toxicity in neurons, and it can prevent the oxidative damage. These findings may enable the development of novel therapeutic strategies for neurological diseases.




Similar content being viewed by others
References
Chen H, Firestein BL (2007) RhoA regulates dendrite branching in hippocampal neurons by decreasing cypin protein levels. J Neurosci Off J Soc Neurosci 27(31):8378–8386. doi:10.1523/JNEUROSCI.0872-07.2007
Nakayama T, Mizoguchi T, Uehara S, Yamashita T, Kawahara I, Kobayashi Y, Moriyama Y, Kurihara S, Sahara N, Ozawa H, Udagawa N, Takahashi N (2011) Polarized osteoclasts put marks of tartrate-resistant acid phosphatase on dentin slices—a simple method for identifying polarized osteoclasts. Bone 49(6):1331–1339. doi:10.1016/j.bone.2011.09.045
Qin H, Liu T, Yang JL, Huang X, Liu B, Song X, Zhao X, Wei YQ (2007) Screening proteins related to retinoic acid resistance by proteomic analysis. Zhonghua Yi Xue Za Zhi 87(8):520–525
Patenaude A, Deschesnes RG, Rousseau JL, Petitclerc E, Lacroix J, Cote MF, CG R (2007) New soft alkylating agents with enhanced cytotoxicity against cancer cells resistant to chemotherapeutics and hypoxia. Cancer Res 67(5):2306–2316. doi:10.1158/0008-5472.CAN-06-3824
Huang CH, Cheng JC, Chen JC, Tseng CP (2007) Evaluation of the role of Disabled-2 in nerve growth factor-mediated neurite outgrowth and cellular signalling. Cell Signal 19(6):1339–1347. doi:10.1016/j.cellsig.2007.01.019
Prabu SM, Shagirtha K, Renugadevi J (2011) Naringenin in combination with vitamins C and E potentially protects oxidative stress-mediated hepatic injury in cadmium-intoxicated rats. J Nutr Sci Vitaminol 57(2):177–185
Haas MA, Vickers JC, Dickson TC (2007) Rho kinase activates ezrin-radixin-moesin (ERM) proteins and mediates their function in cortical neuron growth, morphology and motility in vitro. J Neurosci Res 85(1):34–46. doi:10.1002/jnr.21102
Lukawski K, Nieradko B, Sieklucka-Dziuba M (2005) Effects of cadmium on memory processes in mice exposed to transient cerebral oligemia. Neurotoxicol Teratol 27(4):575–584. doi:10.1016/j.ntt.2005.05.009
Nishimura Y, Yamaguchi JY, Kanada A, Horimoto K, Kanemaru K, Satoh M, Oyama Y (2006) Increase in intracellular Cd(2+) concentration of rat cerebellar granule neurons incubated with cadmium chloride: cadmium cytotoxicity under external Ca(2+)-free condition. Toxicol In Vitro Int J Publ Assoc BIBRA 20(2):211–216. doi:10.1016/j.tiv.2005.06.006
Paul BZ, Kim S, Dangelmaier C, Nagaswami C, Jin J, Hartwig JH, Weisel JW, Daniel JL, Kunapuli SP (2003) Dynamic regulation of microtubule coils in ADP-induced platelet shape change by p160ROCK (Rho-kinase). Platelets 14(3):159–169
Okuda B, Iwamoto Y, Tachibana H, Sugita M (1997) Parkinsonism after acute cadmium poisoning. Clin Neurol Neurosurg 99(4):263–265
Azad MB, Chen Y, Gibson SB (2009) Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal 11(4):777–790. doi:10.1089/ARS.2008.2270
Scherz-Shouval R, Elazar Z (2011) Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci 36(1):30–38. doi:10.1016/j.tibs.2010.07.007
Hu Y, Cui Q, Harvey AR (2007) Interactive effects of C3, cyclic AMP and ciliary neurotrophic factor on adult retinal ganglion cell survival and axonal regeneration. Mol Cell Neurosci 34(1):88–98. doi:10.1016/j.mcn.2006.10.005
Lee J, Giordano S, Zhang J (2012) Autophagy, mitochondria and oxidative stress: cross-talk and redox signalling. Biochem J 441(2):523–540. doi:10.1042/BJ20111451
Luo YH, Wu SB, Wei YH, Chen YC, Tsai MH, Ho CC, Lin SY, Yang CS, Lin P (2013) Cadmium-based quantum dot induced autophagy formation for cell survival via oxidative stress. Chem Res Toxicol 26(5):662–673. doi:10.1021/tx300455k
Fukushima N, Morita Y (2006) Actomyosin-dependent microtubule rearrangement in lysophosphatidic acid-induced neurite remodeling of young cortical neurons. Brain Res 1094(1):65–75. doi:10.1016/j.brainres.2006.04.007
Shimoyama T, Hamano T, Natsume T, Koizumi F, Kiura K, Tanimoto M, Nishio K (2006) Reference profiling of the genomic response induced by an antimicrotubule agent, TZT-1027 (Soblidotin), in vitro. Pharmacogenomics J 6(6):388–396. doi:10.1038/sj.tpj.6500386
Ipe BI, Lehnig M, Niemeyer CM (2005) On the generation of free radical species from quantum dots. Small 1(7):706–709. doi:10.1002/smll.200500105
Gorovoy M, Niu J, Bernard O, Profirovic J, Minshall R, Neamu R, Voyno-Yasenetskaya T (2005) LIM kinase 1 coordinates microtubule stability and actin polymerization in human endothelial cells. J Biol Chem 280(28):26533–26542. doi:10.1074/jbc.M502921200
Rego AC, Oliveira CR (2003) Mitochondrial dysfunction and reactive oxygen species in excitotoxicity and apoptosis: implications for the pathogenesis of neurodegenerative diseases. Neurochem Res 28(10):1563–1574
Floyd RA (1999) Neuroinflammatory processes are important in neurodegenerative diseases: an hypothesis to explain the increased formation of reactive oxygen and nitrogen species as major factors involved in neurodegenerative disease development. Free Radic Biol Med 26(9–10):1346–1355
Wei X, Qi Y, Zhang X, Gu X, Cai H, Yang J, Zhang Y (2015) ROS act as an upstream signal to mediate cadmium-induced mitophagy in mouse brain. Neurotoxicology 46:19–24. doi:10.1016/j.neuro.2014.11.007
Scherz-Shouval R, Shvets E, Fass E, Shorer H, Gil L, Elazar Z (2007) Reactive oxygen species are essential for autophagy and specifically regulate the activity of Atg4. EMBO J 26(7):1749–1760. doi:10.1038/sj.emboj.7601623
Okai T, Araki Y, Tada M, Tateno T, Kontani K, Katada T (2004) Novel small GTPase subfamily capable of associating with tubulin is required for chromosome segregation. J Cell Sci 117(Pt 20):4705–4715. doi:10.1242/jcs.01347
Boutillier S, Rapp J, Staeb T, Olenik C, Schmidt G, Meyer DK, Leemhuis J (2003) Cytotoxic necrotizing factor-2 of Escherichia coli alters the morphology of cultured hippocampal neurons. Naunyn Schmiedeberg’s Arch Pharmacol 368(6):513–519. doi:10.1007/s00210-003-0830-4
Buttner B, Kannicht C, Reutter W, Horstkorte R (2003) The neural cell adhesion molecule is associated with major components of the cytoskeleton. Biochem Biophys Res Commun 310(3):967–971
Nambu H, Nambu R, Melia M, Campochiaro PA (2003) Combretastatin A-4 phosphate suppresses development and induces regression of choroidal neovascularization. Invest Ophthalmol Vis Sci 44(8):3650–3655
Pankiv S, Clausen TH, Lamark T, Brech A, Bruun JA, Outzen H, Overvatn A, Bjorkoy G, Johansen T (2007) p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem 282(33):24131–24145. doi:10.1074/jbc.M702824200
Nishida Y, Arakawa S, Fujitani K, Yamaguchi H, Mizuta T, Kanaseki T, Komatsu M, Otsu K, Tsujimoto Y, Shimizu S (2009) Discovery of Atg5/Atg7-independent alternative macroautophagy. Nature 461(7264):654–658. doi:10.1038/nature08455
Komatsu M, Waguri S, Chiba T, Murata S, Iwata J, Tanida I, Ueno T, Koike M, Uchiyama Y, Kominami E, Tanaka K (2006) Loss of autophagy in the central nervous system causes neurodegeneration in mice. Nature 441(7095):880–884. doi:10.1038/nature04723
Sun K, Xie X, Liu Y, Han Z, Zhao X, Cai N, Zhang S, Song J, Wei L (2013) Autophagy lessens ischemic liver injury by reducing oxidative damage. Cell Biosci 3(1):26. doi:10.1186/2045-3701-3-26
Yuan Y, Jiang CY, Xu H, Sun Y, Hu FF, Bian JC, Liu XZ, Gu JH, Liu ZP (2013) Cadmium-induced apoptosis in primary rat cerebral cortical neurons culture is mediated by a calcium signaling pathway. PLoS One 8(5), e64330. doi:10.1371/journal.pone.0064330
Sakamoto M, Kondo A, Kawasaki K, Goto T, Sakamoto H, Miyake K, Koyamatsu Y, Akiya T, Iwabuchi H, Muroya T, Ochiai K, Tanaka T, Kikuchi Y, Tenjin Y (2001) Analysis of gene expression profiles associated with cisplatin resistance in human ovarian cancer cell lines and tissues using cDNA microarray. Hum Cell Off J Hum Cell Res Soc 14(4):305–315
Di Gioacchino M, Petrarca C, Perrone A, Farina M, Sabbioni E, Hartung T, Martino S, Esposito DL, Lotti LV, Mariani-Costantini R (2008) Autophagy as an ultrastructural marker of heavy metal toxicity in human cord blood hematopoietic stem cells. Sci Total Environ 392(1):50–58. doi:10.1016/j.scitotenv.2007.11.009
Del Pino J, Zeballos G, Anadon MJ, Capo MA, Diaz MJ, Garcia J, Frejo MT (2014) Higher sensitivity to cadmium induced cell death of basal forebrain cholinergic neurons: a cholinesterase dependent mechanism. Toxicology 325:151–159. doi:10.1016/j.tox.2014.09.004
Wang B, Du Y (2013) Cadmium and its neurotoxic effects. Oxidative Med Cell Longev 2013:898034. doi:10.1155/2013/898034
Chen L, Xu B, Liu L, Luo Y, Zhou H, Chen W, Shen T, Han X, Kontos CD, Huang S (2011) Cadmium induction of reactive oxygen species activates the mTOR pathway, leading to neuronal cell death. Free Radic Biol Med 50(5):624–632. doi:10.1016/j.freeradbiomed.2010.12.032
Yu Y, Duan J, Yu Y, Li Y, Liu X, Zhou X, Ho KF, Tian L, Sun Z (2014) Silica nanoparticles induce autophagy and autophagic cell death in HepG2 cells triggered by reactive oxygen species. J Hazard Mater 270:176–186. doi:10.1016/j.jhazmat.2014.01.028
Zhao Z, Han F, Yang S, Wu J, Zhan W (2015) Oxamate-mediated inhibition of lactate dehydrogenase induces protective autophagy in gastric cancer cells: involvement of the Akt-mTOR signaling pathway. Cancer Lett 358(1):17–26. doi:10.1016/j.canlet.2014.11.046
Kroemer G, Jaattela M (2005) Lysosomes and autophagy in cell death control. Nat Rev Cancer 5(11):886–897. doi:10.1038/nrc1738
Wang Q, Zhu J, Zhang K, Jiang C, Wang Y, Yuan Y, Bian J, Liu X, Gu J, Liu Z (2013) Induction of cytoprotective autophagy in PC-12 cells by cadmium. Biochem Biophys Res Commun 438(1):186–192. doi:10.1016/j.bbrc.2013.07.050
Acknowledgments
This work was supported by the National Natural Science Foundation of China (31302058, 31101866, 31172373), a Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We are thankful to Dr Maozhi Hu for technical assistance in flow cytometry analysis.
Conflict of Interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, T., Wang, Q., Song, R. et al. Autophagy Plays a Cytoprotective Role During Cadmium-Induced Oxidative Damage in Primary Neuronal Cultures. Biol Trace Elem Res 168, 481–489 (2015). https://doi.org/10.1007/s12011-015-0390-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-015-0390-8