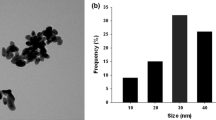
Abstract
Copper oxide nanoparticles (CuONPs) are widely used in several products and their release into the environment can cause toxicity to major food crops. In this study, toxic responses as a result of CuONPs exposure were studied in soybean (Glycine max L.) seedlings. The plants were grown in 1/2 strength Murashige and Skoog medium containing 0, 50, 100, 200, 400, and 500 mg/L of CuONPs in a growth chamber at 26 ± 2 °C with 16/8 h light/dark photoperiod for 14 days. The toxic effects of CuONPs were tested on the shoot and root development, total chlorophyll content, hydrogen peroxide generation, peroxidase (POD) enzyme activity, and lignification of root cells. The mRNA expression of different genes involved in lignin biosynthesis viz. phenylalanine ammonia lyase (PAL), cinnamate 4-hydroxylase (C4H), cinnamyl alcohol dehydrogenase (CAD), peroxidase 2 (POD2), peroxidase 4 (POD4), and peroxidase 7 (POD7) was studied using real-time polymerase chain reaction. Exposure to 500 mg/L of CuONPs significantly reduced the shoot growth, weight, and total chlorophyll content. However, the root length and fresh weights were significantly reduced at all concentrations of CuONPs exposure. Exposure to 100, 200, 400, and 500 mg/L of CuONPs significantly increased the hydrogen peroxide level, POD activity, and lignin contents of roots. Treatment with 2,7-dichlorofluorescein diacetate indicated a concentration-dependent increase in reactive oxygen species generation in roots. Staining with phloroglucinol-HCl revealed a concentration dependant increase in lignification of root cells. The expression levels of PAL, C4H, and CAD genes were significantly up-regulated upon exposure to 100, 200, and 400 mg/L of CuONPs. Significant up-regulation in the expression levels of POD2 and POD4 genes was observed upon exposure to 100, 200, 400, and 500 mg/L of CuONPs. Exposure to 200, 400, and 500 mg/L of CuONPs resulted in significant up-regulation of POD7 gene. These results for the first time show that exposure to CuONPs causes enhanced lignification of root cells and thereby affect root development in soybean seedlings.







Similar content being viewed by others
References
Chen Y, Wang D, Zhu X, Zheng X, Feng L (2012) Long-term effects of copper nanoparticles on wastewater biological nutrient removal and N2O generation in the activated sludge process. Environ Sci Technol 46:12452–12458
Gottschalk F, Nowack B (2011) The release of engineered nanomaterials to the environment. J Environ Monit 13:1145–1155
Brar SK, Verma M, Tyagi RD, Surampalli RY (2010) Engineered nanoparticles in waste water and waste water sludge-evidence and impacts. Waste Manag 30:504–520
Oleszczuk P, Josko I, Xing BS (2011) The toxicity to plants of the sewage sludges containing multiwalled carbon nanotubes. J Hazard Mater 186:436–442
Lee WM, An YJ, Yoon H, Kweon HS (2008) Toxicity and bioavailability of copper nanoparticles to the terrestrial plants mung bean (Phaseolus radiatus) and wheat (Triticum aestivum): plant agar test for water-insoluble nanoparticles. Environ Toxicol Chem 27:1915–1921
Dimkpa CO, McLean JE, Martineau N, Britt DW, Haverkamp R, Anderson AJ (2013) Silver nanoparticles disrupt wheat (Triticum aestivum L.) growth in a sand matrix. Environ Sci Technol 47:1082–1090
Shaw AK, Hossain Z (2013) Impact of nano-CuO stress on rice (Oryza sativa L.) seedlings. Chemosphere 93:906–915
Lin CC, Chen LM, Liu ZH (2005) Rapid effect of copper on lignin biosynthesis in soybean roots. Plant Sci 168:855–861
Xu Z, Zhang D, Hu J, Zhou X et al (2009) Comparative genome analysis of lignin biosynthesis gene families across the plant kingdom. BMC Bioinforma 10(Suppl 11):S3
Welinder KG (1992) Superfamily of plant, fungal and bacterial peroxidases. Curr Opin Struct Biol 2:388–393
Ma¨der M, Fu¨ssl R (1982) Role of peroxidase in lignification of tobacco cells: regulation by phenolic compounds. Plant Physiol 70:1132–1134
Yruela I (2005) Copper in plants. Braz J Plant Physiol 17:145–146
Xiong ZT, Wang H (2005) Copper toxicity and bioaccumulation in Chinese cabbage (Brassica pekiensis Rupr.). Environ Toxicol 20:188–194
Lequeux H, Hermans C, Lutts S, Nathalie V (2010) Response to copper excess in Arabidopsis thaliana: impact on the root system architecture, hormone distribution, lignin accumulation and mineral profile. Plant Physiol Biochem 48:673–682
Finger-Teixeira A, Ferrarese Mde L, Soares AR, da Silva D, Ferrarese-Filho O (2010) Cadmium-induced lignification restricts soybean root growth. Ecotoxicol Environ Saf 73:1959–1964
Polle A, Otter T, Seifert F (1994) Apoplastic peroxidase and lignification in needles of Norway spruce (Picea abies L.). Plant Physiol 106:53–60
Klotz KL, Liu TTY, Liu L, Largimini L (1998) Expression of the tobacco anionic peroxidase gene is tissue-specific and development regulated. Plant Mol Biol 36:509–520
Nair PMG, Chung IM (2014) Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignification and molecular level changes. Environ Sci Pollut Res. doi:10.1007/s11356-014-3210-3
USEPA (U.S.Environmental Protection Agency), 1996. Ecological effects test guide-lines: seed germination/root elongation toxicity test, OPPTS850.4200. EPA, Washington
Rodríguez-Serrano M, Romero-Puertas MC, Zabalza A, Corpas FJ, Gómez M, del Río LA, Sandalio LM (2006) Cadmium effect on oxidative metabolism of pea (Pisum sativum L.) roots: imaging of reactive oxygen species and nitric oxide accumulation in vivo. Plant Cell Environ 29:1532–1544
Jana S, Choudhuri MA (1982) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquat Bot 12:345–354
Rogers LA, Dubos C, Surman C, Willment J, Cullis IF, Mansfield SD, Campbell MM (2005) Comparison of lignin deposition in three ectopic lignification mutants. New Phytol 168:123–140
OECD (Organization for Economic Cooperation and Development) (2004) Proposal for updating guideline 208. Terrestrial plant test: seedling emergence and seedling growth test. Draft document
Wu SG, Huang L, Head J, Chen DR, Kong IC, Tang YJ (2012) Phytotoxicity of metal oxide nanoparticles is related to both dissolved metals ions and adsorption of particles on seed surfaces. J Pet Environ Biotechnol 3:1000126
Adhikari T, Kundu S, Biswas AK, Tarafdar JC, Rao AS (2012) Effect of copper oxide nano particle on seed germination of selected crops. J Agri Sci Technol A2:815–823
Shi J, Peng C, Yang Y, Yang J, Zhang H, Yuan X, Chen Y, Hu T (2014) Phytotoxicity and accumulation of copper oxide nanoparticles to the Cu-tolerant plant Elsholtzia splendens. Nanotoxicol 8:179–188
Wang Z, Xie X, Zhao J, Liu X, Feng W, White JC, Xing B (2012) Xylem and phloem based transport of CuO nanoparticles in maize (Zea mays L.). Environ Sci Technol 46:4434–4441
Takeshi M, In Sook ML, Noriyuki S, Takakiro I (1997) Identification of S100b protein as copper-binding protein and its suppression of copper-induced cell damage. J Biol Chem 272:23037–23041
Sandmann G, Boger P (1980) Copper-mediated lipid peroxidation processes in photosynthetic membranes. Plant Physiol 66:797–800
Passardi F, Cosio C, Penel C, Dunand C (2005) Peroxidases have more functions than a Swiss army knife. Plant Cell Rep 24:255–265
Baldoni A, Von Pinho EVR, Fernandes JS, Abreu VM, Carvalho MLM (2013) Gene expression in the lignin biosynthesis pathway during soybean seed development. Genet Mol Res 12:2618–2624
Raes J, Rohde A, Christensen JH, Van de Peer Y et al (2003) Genome-wide characterization of the lignification tool box in Arabidopsis. Plant Physiol 133:1051–1071
Lagrimini LM (1991) Wound-induced deposition of polyphenols in transgenic plants over expressing peroxidase. Plant Physiol 96:577–583
Quiroga M, Guerrero C, Botella MA, Barcelo A, Amaya I, Medina MI, Alonso FJ, Forchetti SM, Tigier H, Valpuesta V (2000) A tomato peroxidase involved in the synthesis of lignin and suberin. Plant Physiol 122:1119–1127
Mazhoudi S, Chaoui A, Ghorbal MH, Elferjani E (1997) Response of antioxidant enzyme to excess copper in tomato (Lycopersicon esculentum Mill). Plant Sci 127:129–137
Jouili H, Ezzedine EF (2003) Changes in antioxidant and lignifying enzyme activities in sunflower roots (Helianthus annuus L.) stressed with copper excess. C R Biol 326:639–644
Santos WDD, Ferrarese MLL, Nakamura CV, Mourão KSM, Mangolin CA, Ferrarese-Filho O (2008) Soybean (Glycine max) root lignification induced by ferulic acid. The possible mode of action. J Chem Ecol 34:1230–1241
Fielding JL, Hall JL (1978) A biochemical and cytochemical study of peroxidase activity in roots of Pisum sativum. J Exp Bot 29:969–981
Gaspar T, Penel C, Castillo FJ, Greppin H (1985) A two step control of basic and acidic peroxidases and its significance for growth and development. Physiol Plant 64:418–423
Castillo FJ (1986) Extracellular peroxidases as markers of stress? In: Greppin H, Penel C, Gaspar T (eds) Molecular and physiological aspects of plant peroxidases. University of Geneva Press, Geneva, pp 419–426
Lamport DTA (1986) Roles for peroxidases in cell wall genesis. In: Greppin H, Penel C, Gaspar T (eds) Molecular and physiological aspects of plant peroxidases. University of Geneva Press, Geneva, pp 199–208
Sanchez M, Revilla G, Zarra I (1995) Changes in peroxidase activity associated with cell walls during pine hypocotyl growth. Ann Bot 75:415–419
Li TC, Feg TY, Chen WS, Liu ZH (2001) The acute effect of copper on the levels of indole-3-acetic acid and lignin in peanut roots. Aust J Plant Physiol 28:1–6
Acknowledgments
This paper was supported by the KU Research Professor Program of Konkuk University.
Conflict of Interest
The authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Nair, P.M.G., Chung, I.M. A Mechanistic Study on the Toxic Effect of Copper Oxide Nanoparticles in Soybean (Glycine max L.) Root Development and Lignification of Root Cells. Biol Trace Elem Res 162, 342–352 (2014). https://doi.org/10.1007/s12011-014-0106-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-014-0106-5