Abstract
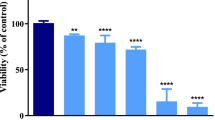
As a representative element of lanthanide, lanthanum has been widely used in various fields and eventually entered environment and accumulated in human body. Epidemiological and experimental evidences indicated that lanthanum has neurotoxicity; however, the detailed mechanism is still elusive. Here, we chose primary cerebral cortical neurons as model in vitro to investigate the mechanism underlying the toxic effects of lanthanum chloride (LaCl3). This study revealed the following findings: (1) LaCl3 treatment (0.01, 0.1, and 1.0 mM for 24 h) reduced the viability of cortical neurons and elevated apoptotic rate significantly in a dose-dependent manner. (2) LaCl3 triggered mitochondrial apoptotic pathway in cortical neurons, characterized with collapsed mitochondrial membrane potential, release of cytochrome c into cytosol, and increasing expression of activated caspase-3. (3) LaCl3 elevated intracellular Ca2+ concentration, promoted reactive oxygen species generation, and upregulated pro-apoptotic Bax, whereas it downregulated anti-apoptotic Bcl-2 expression and consequently altered Bax/Bcl-2 ratio, which ultimately lead to neuronal mitochondrial apoptosis. Our results demonstrated that toxicity of lanthanum in cortical neurons perhaps partly attributed to enhanced mitochondrial apoptosis due to mitochondrial dysfunction modulated by Ca2+ and Bcl-2 family.






Similar content being viewed by others
References
He X, Zhang Z, Zhang H, Zhao Y, Chai Z (2008) Neurotoxicological evaluation of long-term lanthanum chloride exposure in rats. Toxicol Sci 103:354–361
D’Haese PC, Spasovski GB, Sikole A, Hutchison A, Freemont TJ, Sulkova S, Swanepoel C, Pejanovic S, Djukanovic L, Balducci A, Coen G, Sulowicz W, Ferreira A, Torres A, Curic S, Popovic M, Dimkovic N, De Broe ME (2003) A multicenter study on the effects of lanthanum carbonate (Fosrenol) and calcium carbonate on renal bone disease in dialysis patients. Kidney Int Suppl 85:S73–S78
Fan G, Yuan Z, Zheng H, Liu Z (2004) Study on the effects of exposure to rare earth elements and health-responses in children aged 7–10 years. Wei Sheng Yan Jiu 33:23–28
Briner W, Rycek RF, Moellenberndt A, Dannull K (2000) Neurodevelopmental effects of lanthanum in mice. Neurotoxicol Teratol 22:573–581
Che Y, Xing R, Zhu Y, Cui Y, Jiang X (2010) Effects of lanthanum chloride administration on detouring learning in chicks. Biol Trace Elem Res 143:274–280
Cheng Z, Li N, Cheng J, Hu R, Gao G, Cui Y, Gong X, Wang L, Hong F (2012) Signal pathway of hippocampal apoptosis and cognitive impairment of mice caused by cerium chloride. Environ Toxicol 27:707–718
Feng L, Xiao H, He X, Li Z, Li F, Liu N, Zhao Y, Huang Y, Zhang Z, Chai Z (2006) Neurotoxicological consequence of long-term exposure to lanthanum. Toxicol Lett 165:112–120
Zhao H, Cheng Z, Cheng J, Hu R, Che Y, Cui Y, Wang L, Hong F (2011) The toxicological effects in brain of mice following exposure to cerium chloride. Biol Trace Elem Res 144:872–884
Zhao H, Cheng Z, Hu R, Chen J, Hong M, Zhou M, Gong X, Wang L, Hong F (2011) Oxidative injury in the brain of mice caused by lanthanid. Biol Trace Elem Res 142:174–189
Antonsson B (2004) Mitochondria and the bcl-2 family proteins in apoptosis signaling pathways. Mol Cell Biochem 256–257:141–155
Oliver L, Vallette FM (2005) The role of caspases in cell death and differentiation. Drug Resist Updat 8:163–170
Yuan J, Yankner BA (2000) Apoptosis in the nervous system. Nature 407:802–809
Harrington HA, Ho KL, Ghosh S, Tung KC (2008) Construction and analysis of a modular model of caspase activation in apoptosis. Theor Biol Med Model 5:26
Antonsson B (2001) Bax and other pro-apoptotic bcl-2 family “killer-proteins” and their victim the mitochondrion. Cell Tissue Res 306:347–361
Soane L, Fiskum G (2005) Inhibition of mitochondrial neural cell death pathways by protein transduction of bcl-2 family proteins. J Bioenerg Biomembr 37:179–190
Caroppi P, Sinibaldi F, Fiorucci L, Santucci R (2009) Apoptosis and human diseases: mitochondrion damage and lethal role of released cytochrome c as proapoptotic protein. Curr Med Chem 16:4058–4065
Petrosillo G, Ruggiero FM, Pistolese M, Paradies G (2004) Ca2+-induced reactive oxygen species production promotes cytochrome c release from rat liver mitochondria via mitochondrial permeability transition (mpt)-dependent and mpt-independent mechanisms: role of cardiolipin. J Biol Chem 279:53103–53108
Liu SS, Lu D, Miao LF, Xiong QY, Chen XP, Wang Y, Guo F (2010) Effects of lanthanum chloride on proliferation and migration of human cervical cancer cell line HeLa cells. Zhonghua Fu Chan Ke Za Zhi 45:609–613
Shen L, Lan Z, Sun X, Shi L, Liu Q, Ni J (2010) Proteomic analysis of lanthanum citrate-induced apoptosis in human cervical carcinoma SiHa cells. BioMetals 23:1179–1189
Ji YJ, Xiao B, Wang ZH, Cui MZ, Lu YY (2000) The suppression effect of light rare earth elements on proliferation of two cancer cell lines. Biomed Environ Sci 13:287–292
Yang J, Q Liu, M Qi, S Lu, S Wu, Q Xi, Y Cai (2013) Lanthanum chloride promotes mitochondrial apoptotic pathway in primary cultured rat astrocytes. Environ Toxicol. doi:10.1002/tox.20738
Gramowski A, Jugelt K, Schroder OHU, Weiss DG, Mitzner S (2010) Acute functional neurotoxicity of lanthanum(III) in primary cortical networks. Toxicol Sci 120:173–183
Dawson VL, Dawson TM, London ED, Bredt DS, Snyder SH (1991) Nitric oxide mediates glutamate neurotoxicity in primary cortical cultures. Proc Natl Acad Sci U S A 88:6368–6371
Li Chun-li Y et al (2011) Primary culture method and identify in neonatal rat hippocampal and cortical neurons. J of Shenyang Pharm Univ 28:299–304
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Watjen W, Cox M, Biagioli M, Beyersmann D (2002) Cadmium-induced apoptosis in c6 glioma cells: mediation by caspase 9-activation. BioMetals 15:15–25
Rothe G, Valet G (1990) Flow cytometric analysis of respiratory burst activity in phagocytes with hydroethidine and 2′,7′-dichlorofluorescin. J Leukoc Biol 47:440–448
Grynkiewicz G, Poenie M, Tsien RY (1985) A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Jekabsone A, Dapkunas Z, Brown GC, Borutaite V (2003) S-nitrosothiol-induced rapid cytochrome c release, caspase activation and mitochondrial permeability transition in perfused heart. Biochem Pharmacol 66:1513–1519
Jin C, Wu S, Lu X, Liu Q, Qi M, Lu S, Xi Q, Cai Y (2011) Induction of the bystander effect in Chinese hamster v79 cells by actinomycin D. Toxicol Lett 202:178–185
Antonsson B (2004) Mitochondria and bcl-2 family proteins in apoptosis signaling pathway. Mol Cell Biochem 256(257):141–155
Katunuma N, Matsui A, Le QT, Utsumi K, Salvesen G, Ohashi A (2001) Novel procaspase-3 activating cascade mediated by lysoapoptases and its biological significances in apoptosis. Adv Enzym Regul 41:237–250
Ott M, Gogvadze V, Orrenius S, Zhivotovsky B (2007) Mitochondria, oxidative stress and cell death. Apoptosis 12:913–922
Xia Z, Lundgren B, Bergstrand A, DePierre JW, Nassberger L (1999) Changes in the generation of reactive oxygen species and in mitochondrial membrane potential during apoptosis induced by the antidepressants imipramine, clomipramine, and citalopram and the effects on these changes by bcl-2 and bcl-x(l). Biochem Pharmacol 57:1199–1208
Jiang J, Huang Z, Zhao Q, Feng W, Belikova NA, Kagan VE (2008) Interplay between bax, reactive oxygen species production, and cardiolipin oxidation during apoptosis. Biochem Biophys Res Commun 368:145–150
Walters J et al (2009) A constitutively active and uninhibitable caspase-3 zymogen efficiently induces apoptosis. Biochem J 424:335–345
Shen L, Liu Q, Ni J, Hong G (2009) A proteomic investigation into the human cervical cancer cell line HeLa treated with dicitratoytterbium (III) complex. Chem Biol Interact 181:455–462
Dykens JA (1994) Isolated cerebral and cerebellar mitochondria produce free radicals when exposed to elevated Ca2+ and Na+: implications for neurodegeneration. J Neurochem 63:584–591
Fiskum G (2000) Mitochondrial participation in ischemic and traumatic neural cell death. J Neurotrauma 17:843–855
Brookes PS (2004) Calcium, ATP, and ROS: a mitochondrial love-hate triangle. AJP: Cell Physiol 287:C817–C833
Liu H, Yuan L, Yang X, Wang K (2003) La(3+), Gd(3+) and Yb(3+) induced changes in mitochondrial structure, membrane permeability, cytochrome c release and intracellular ROS level. Chem Biol Interact 146:27–37
Merry DE, Korsmeyer SJ (1997) Bcl-2 gene family in the nervous system. Annu Rev Neurosci 20:245–267
Mizuta T, Shimizu S, Matsuoka Y, Nakagawa T, Tsujimoto Y (2007) A bax/bak-independent mechanism of cytochrome c release. J Biol Chem 282:16623–16630
Sagot Y, Dubois-Dauphin M, Tan SA, de Bilbao F, Aebischer P, Martinou JC, Kato AC (1995) Bcl-2 overexpression prevents motoneuron cell body loss but not axonal degeneration in a mouse model of a neurodegenerative disease. J Neurosci 15:7727–7733
Gagliardini V, Fernandez PA, Lee RK, Drexler HC, Rotello RJ, Fishman MC, Yuan J (1994) Prevention of vertebrate neuronal death by the crma gene. Science 263:826–828
Martinou JC, Dubois-Dauphin M, Staple JK, Rodriguez I, Frankowski H, Missotten M, Albertini P, Talabot D, Catsicas S, Pietra C et al (1994) Overexpression of bcl-2 in transgenic mice protects neurons from naturally occurring cell death and experimental ischemia. Neuron 13:1017–1030
Polster BM, Kinnally KW, Fiskum G (2001) Bh3 death domain peptide induces cell type-selective mitochondrial outer membrane permeability. J Biol Chem 276:37887–37894
Kowaltowski AJ, Vercesi AE, Fiskum G (2000) Bcl-2 prevents mitochondrial permeability transition and cytochrome c release via maintenance of reduced pyridine nucleotides. Cell Death Differ 7:903–910
Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, Roth KA, MacGregor GR, Thompson CB, Korsmeyer SJ (2001) Proapoptotic bax and bak: a requisite gateway to mitochondrial dysfunction and death. Science 292:727–730
Korsmeyer SJ (1992) Bcl-2: an antidote to programmed cell death. Cancer Surv 15:105–118
Acknowledgments
This study was supported by the National Natural Science Foundation of China (grant nos. 81072316 and 30800925) and Science Project of Education Department in Province Liaoning, China (grant nos. L2010702 and L2012290).
Conflict of interest
The authors declare there is no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Author Jie Wu and Author Jinghua Yang contributed equally to this study.
Rights and permissions
About this article
Cite this article
Wu, J., Yang, J., Liu, Q. et al. Lanthanum Induced Primary Neuronal Apoptosis Through Mitochondrial Dysfunction Modulated by Ca2+ and Bcl-2 Family. Biol Trace Elem Res 152, 125–134 (2013). https://doi.org/10.1007/s12011-013-9601-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12011-013-9601-3