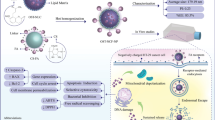
Abstract
In this study, chitosan-lecithin nanoparticles modified with polyethylene glycol (PEG) and folic acid (FA) were used to deliver allicin (AC) to colon cancer cells. AC-loaded polyethylene glycol (PEG) and folic acid (FA)-modified chitosan-lecithin nanoparticles (AC-PLCF-NPs) were fabricated via self-assembling procedure. HPLC for AC encapsulation and FA binding, MTT for viability assay, ABTS and DPPH for antioxidant capacity, disc diffusion, MIC and MBC for antibacterial assay, qPCR and AO/PI staining for apoptotic, and CAM assay for angiogenesis effects of AC-PLCF-NPs were used. AC-PLCF-NPs (113.55 nm) were synthesized as single dispersed (PDI: 0.28) and stable (ZP: + 33.18 mV) with 81% AC encapsulation and 48% FA binding. The antioxidant power of AC-PLCF-NPs was confirmed by inhibiting free radicals ABTS (74.25 µg/mL) and DPPH (366.214 µg/mL) and its antibacterial capacity with very high inhibitory effects against gram-negative bacterial strains. MTT results showed higher toxicity of AC-PLCF-NPs (68.06 µg/mL) compared to AC (171.45 µg/mL). Increased expression of caspase 3 and 9 genes showed activation of the intrinsic apoptosis pathway in treated cells, and on the other hand, reduction of vascular and embryonic growth factors in CAM model confirmed the anti-angiogenesis effects of AC-PLCF-NPs. AC-PLCF-NPs can be suggested as a promising therapeutic agent for studies in the field of colon cancer treatment.








Similar content being viewed by others
Data Availability
Not applicable.
References
Al-Ishaq, R. K., Overy, A. J., & Büsselberg, D. (2020). Phytochemicals and gastrointestinal cancer: Cellular mechanisms and effects to change cancer progression. Biomolecules, 10(1), 105.
Sarvizadeh, M., et al. (2021). Allicin and digestive system cancers: From chemical structure to its therapeutic opportunities. Frontiers in Oncology, 563.
Lau, H. C. H., et al. (2020). Organoid models of gastrointestinal cancers in basic and translational research. Nature reviews Gastroenterology & hepatology, 17(4), 203–222.
Sitarz, R., et al. (2018). Gastric cancer: Epidemiology, prevention, classification, and treatment. Cancer Management Research, 10, 239–248.
Pourhanifeh, M. H., et al. (2020). Autophagy-related microRNAs: Possible regulatory roles and therapeutic potential in and gastrointestinal cancers. Pharmacological Research, 161, 105133.
Parikh, A. R., et al. (2020). Serial ctDNA monitoring to predict response to systemic therapy in metastatic gastrointestinal cancers. Clinical Cancer Research, 26(8), 1877–1885.
Moriarty, R. M., Naithani, R., & Surve, B. (2007). Organosulfur compounds in cancer chemoprevention. Mini Reviews in Medicinal Chemistry, 7(8), 827–838.
Nagini, S. (2008). Cancer chemoprevention by garlic and its organosulfur compounds-panacea or promise? Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 8(3), 313–321.
Walag, A. M. P., et al. (2020). Health benefits of organosulfur compounds. Functional Foods and Nutraceuticals 445–472. Springer.
Xiao, D., et al. (2005). Effects of a series of organosulfur compounds on mitotic arrest and induction of apoptosis in colon cancer cells. Molecular Cancer Therapeutics, 4(9), 1388–1398.
Miękus, N., et al. (2020). Health benefits of plant-derived sulfur compounds, glucosinolates, and organosulfur compounds. Molecules, 25(17), 3804.
El-Bayoumy, K., et al. (2006). Cancer chemoprevention by garlic and garlic-containing sulfur and selenium compounds. The Journal of Nutrition, 136(3), 864S-869S.
Shafeeque, K., & Hashim, K. (2018). Comparative anti-angiogenesis study between allicin nanoparticle and normal allicin from garlic (Allium sativum Linn). Europe Journal of Experimental Biology, 8, 27.
Lang, A., et al. (2004). Allicin inhibits spontaneous and TNF-α induced secretion of proinflammatory cytokines and chemokines from intestinal epithelial cells. Clinical Nutrition, 23(5), 1199–1208.
Sun, L., & Wang, X. (2003). Effects of allicin on both telomerase activity and apoptosis in gastric cancer SGC-7901 cells. World Journal of Gastroenterology, 9(9), 1930.
Arditti, F. D., et al. (2005). Apoptotic killing of B-chronic lymphocytic leukemia tumor cells by allicin generated in situ using a rituximab-alliinase conjugate. Molecular Cancer Therapeutics, 4(2), 325–332.
Jakubikova, J., & Sedlak, J. (2006). Garlic-derived organosulfides induce cytotoxicity, apoptosis, cell cycle arrest and oxidative stress in human colon carcinoma cell lines. Neoplasma, 53(3), 191–199.
Zarei, B., Tabrizi, M. H., & Rahmati, A. (2022). PEGylated lecithin-chitosan nanoparticle–encapsulated alphα-terpineol for in vitro anticancer effects. An Official Journal of the American Association of Pharmaceutical Scientists, 23(4), 1–14.
Perez-Ruiz, A. G., et al. (2018). Lecithin–chitosan–TPGS nanoparticles as nanocarriers of (−)-epicatechin enhanced its anticancer activity in breast cancer cells. RSC Advances, 8(61), 34773–34782.
Khan, M. M., et al. (2019). Lipid-chitosan hybrid nanoparticles for controlled delivery of cisplatin. Drug Delivery, 26(1), 765–772.
Mandal, B., et al. (2013). Core–shell-type lipid–polymer hybrid nanoparticles as a drug delivery platform. Nanomedicine: Nanotechnology, Biology and Medicine, 9(4), 474–491.
Rahmati, A., et al. (2022). Fabrication and assessment of folic acid conjugated-chitosan modified PLGA nanoparticle for delivery of alpha terpineol in colon cancer. Journal of Biomaterials Science, Polymer Edition, 33(10), 1289–1307.
Mengoni, T., et al. (2017). A Chitosan—based liposome formulation enhances the in vitro wound healing efficacy of substance P neuropeptide. Pharmaceutics, 9(4), 56.
Kouchakzadeh, H., et al. (2010). Optimization of PEGylation conditions for BSA nanoparticles using response surface methodology. An Official Journal of the American Association of Pharmaceutical Scientists, 11(3), 1206–1211.
Veronese, F. M., & Pasut, G. (2005). PEGylation, successful approach to drug delivery. Drug Discovery Today, 10(21), 1451–1458.
Gavas, S., Quazi, S., & Karpiński, T. M. (2021). Nanoparticles for cancer therapy: Current progress and challenges. Nanoscale Research Letters, 16(1), 1–21.
Hosseini, S. F., et al. (2013). Two-step method for encapsulation of oregano essential oil in chitosan nanoparticles: Preparation, characterization and in vitro release study. Carbohydrate Polymers, 95(1), 50–56.
Salopek, B., Krasic, D., & Filipovic, S. (1992). Measurement and application of zeta-potential. Rudarsko-geolosko-naftni zbornik, 4(1), 147.
Soe, Z. C., et al. (2019). Folate-targeted nanostructured chitosan/chondroitin sulfate complex carriers for enhanced delivery of bortezomib to colorectal cancer cells. Asian Journal of Pharmaceutical Sciences, 14(1), 40–51.
Chen, H., et al. (2018). Allicin inhibits proliferation and invasion in vitro and in vivo via SHP-1-mediated STAT3 signaling in cholangiocarcinoma. Cellular Physiology and Biochemistry, 47(2), 641–653.
Thurston, D.E., & I. Pysz, (2021). Chemistry and pharmacology of anticancer drugs. CRC press. eBook.
Harlev, E., et al. (2012). Anticancer attributes of desert plants: A review. Anti-Cancer Drugs, 23(3), 255–271.
Souid, S., et al. (2017). Allium roseum L. extract exerts potent suppressive activities on chronic myeloid leukemia K562 cell viability through the inhibition of BCR-ABL, PI3K/Akt, and ERK1/2 pathways and the abrogation of VEGF secretion. Nutrition and Cancer, 69(1), 117–130.
Zou, X., et al. (2016). Allicin sensitizes hepatocellular cancer cells to anti-tumor activity of 5-fluorouracil through ROS-mediated mitochondrial pathway. Journal of Pharmacological Sciences, 131(4), 233–240.
Luo, R., et al. (2016). The mechanism in gastric cancer chemoprevention by allicin. Anti-Cancer Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Cancer Agents), 16(7), 802–809.
Oommen, S., et al. (2004). Allicin (from garlic) induces caspase-mediated apoptosis in cancer cells. European Journal of Pharmacology, 485(1–3), 97–103.
Park, S.-Y., et al. (2005). Caspase-independent cell death by allicin in human epithelial carcinoma cells: Involvement of PKA. Cancer Letters, 224(1), 123–132.
Müller, A., et al. (2016). Allicin induces thiol stress in bacteria through S-allylmercapto modification of protein cysteines. Journal of Biological Chemistry, 291(22), 11477–11490.
Ma, Q., et al. (2020). Self-Assembled chitosan/phospholipid nanoparticles: From fundamentals to preparation for advanced drug delivery. Drug Delivery, 27(1), 200–215.
Maiti, R., et al. (2018). Bovine serum albumin nanoparticles constructing procedures on anticancer activities. International Journal of Advanced Research Biology Science, 5(4), 226–239.
Mariyam, M., et al. (2018). Dendrimers: General aspects, applications and structural exploitations as prodrug/drug-delivery vehicles in current medicine. Mini Reviews in Medicinal Chemistry, 18(5), 439–457.
Barbieri, S., et al. (2013). Lecithin/chitosan controlled release nanopreparations of tamoxifen citrate: Loading, enzyme-trigger release and cell uptake. Journal of Controlled Release, 167(3), 276–283.
Hafner, A., et al. (2011). Short- and long-term stability of lyophilised melatonin-loaded lecithin/chitosan nanoparticles. Chemical and Pharmaceutical Bulletin, 59(9), 1117–1123.
Nadeem, M. S., et al. (2021). Allicin, an antioxidant and neuroprotective agent, ameliorates cognitive impairment. Antioxidants, 11(1), 87.
Kelsey, N. A., Wilkins, H. M., & Linseman, D. A. (2010). Nutraceutical antioxidants as novel neuroprotective agents. Molecules, 15(11), 7792–7814.
Kurusu, T., Kuchitsu, K., & Tada, Y. (2015). Plant signaling networks involving Ca2+ and Rboh/Nox-mediated ROS production under salinity stress. Frontiers in Plant Science, 6, 427.
Pendyala, S., & Natarajan, V. (2010). Redox regulation of Nox proteins. Respiratory physiology & neurobiology, 174(3), 265–271.
Gruhlke, M. C., & Slusarenko, A. J. (2012). The biology of reactive sulfur species (RSS). Plant Physiology and Biochemistry, 59, 98–107.
Cutler, R., & Wilson, P. (2004). Antibacterial activity of a new, stable, aqueous extract of allicin against methicillin-resistant Staphylococcus aureus. British Journal of Biomedical Science, 61(2), 71–74.
Zainal, M., et al. (2021). The antimicrobial and antibiofilm properties of allicin against Candida albicans and Staphylococcus aureus–A therapeutic potential for denture stomatitis. The Saudi Dental Journal, 33(2), 105–111.
Abbasi, N., et al. (2021). Cerium oxide nanoparticles-loaded on chitosan for the investigation of anticancer properties. Materials Technology, 37(10), 1439–1449.
Hu, X., et al. (2018). Thymoquinone augments cisplatin-induced apoptosis on esophageal carcinoma through mitigating the activation of JAK2/STAT3 pathway. Digestive Diseases and Sciences, 63(1), 126–134.
Ji, Y., et al. (2012). Angiotensin II induces angiogenic factors production partly via AT1/JAK2/STAT3/SOCS3 signaling pathway in MHCC97H cells. Cellular Physiology and Biochemistry, 29(5–6), 863–874.
Wang, W., et al. (2016). Allicin inhibits lymphangiogenesis through suppressing activation of vascular endothelial growth factor (VEGF) receptor. The Journal of nutritional biochemistry, 29, 83–89.
Teleanu, R. I., et al. (2020). Tumor angiogenesis and anti-angiogenic strategies for cancer treatment. Journal of Clinical Medicine, 9(1), 84.
Maeda, H. (2001). The enhanced permeability and retention (EPR) effect in tumor vasculature: The key role of tumor-selective macromolecular drug targeting. Advances in Enzyme Regulation, 41, 189–207.
Acknowledgements
This work was supported by the Islamic Azad University, Mashhad, Iran, and thus is appreciated by the author.
Funding
This research was performed at personal expense in the laboratory of the Islamic Azad University of Mashhad.
Author information
Authors and Affiliations
Contributions
All authors contributed equally.
Corresponding author
Ethics declarations
Ethical Approval
Not required.
Consent to Participate
All authors have their consent to participate.
Consent to Publish
All authors have their consent to publish their work.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hashemy, S.I., Amiri, H., Hosseini, H. et al. PEGylated Lecithin–Chitosan–Folic Acid Nanoparticles as Nanocarriers of Allicin for In Vitro Controlled Release and Anticancer Effects. Appl Biochem Biotechnol 195, 4036–4052 (2023). https://doi.org/10.1007/s12010-022-04310-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-04310-y