Abstract
Fungal endophytes are considered one of the most important reservoirs of bioactive compounds which defeat resistant microbes. In our study, endophytic Alternaria alternata was isolated from Ziziphus spina-christi and identified morphologically and genetically with accession number OM 331,682. Preliminary phytochemical screening of ethyl acetate (EA) crude extract of A. alternata revealed that this extract contains alkaloids, tannins, flavonoids, glycosides, phenols, and terpenoids. Moreover, the extract was analyzed using gas chromatography-mass spectrometry (GC–MS) which verified the presence of numerous bioactive compounds. Antimicrobial results illustrated that EA crude extract exhibited promising antimicrobial activity against Gram-negative bacteria (Escherichia coli ATCC 11229, Proteus vulgaris RCMB 004, Pseudomonas aeruginosa ATCC 27853, and Klebsiella pneumonia RCMB 003), Gram-positive bacteria (Bacillus subtilis RCMB 015, Staphylococcus aureus ATCC 25923, and Staphylococcus epidermidis ATCC 14990), and unicellular fungi (Candida albicans ATCC 90028). Ultrastructure study of treated K. pneumonia showed remarkably elucidated destruction of the cell wall and cell membrane and leakage of cytoplasmic materials. Furthermore, the extract has potential antioxidant activity where IC50 was 409 µg/mL. Moreover, this extract did not show any toxicity on Vero normal cell line. These findings confirmed that the endophytic A. alternata from Z. spina-christi is a promising source of bioactive compounds which can be used in different biological applications.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The emergence of pathogenic bacteria and fungi resistant to commercial drugs is a relevant problem faced by health services; this is due to the microbes acquiring new mechanisms to resist antimicrobial agents [1, 2]. Therefore, the discovery of effective antimicrobial agents is required. Fungal endophytes can live in plant tissues without producing any apparent symptoms or obvious harm effects to their hosts [3]. They have been existing in all plant species studied [4], colonizing the area underneath the epidermal tissue, absorbing their food from the plants, and improving plant growth of host and protecting it from pathogens by inhibiting the growth of plant pathogen and inducing the systematic resistance of plant as defense mechanisms [5]. Fungal endophytes are considered one of important reservoirs of bioactive compounds which have different biological activities such as antimicrobial, antioxidant, anticancer, antiviral, and antimalarial activities [3, 6, 7]. These activities are attributed to different effective secondary metabolites such as alkaloids, phenols, steroids, terpenoids, saponins, glycosides, tannins, and flavonoids [8, 9]. Endophytic Alternaria spp. have variety of biological activities, such as antimicrobial, antioxidant, antiviral, anticancer, and phytotoxic activities [10, 11]. Recently, more metabolites with different bioactivities from Alternaria fungi have been extracted and structurally characterized. Therefore, exploring of fungal endophytes which live in medicinal plant enables us to discover new metabolites [12]. Fungal endophytes reside in medicinal plant which grows in natural habitat. Stems, leaves, and roots are a huge reservoir for these endophytic fungi which can be used in different biological application as cytotoxic, antibacterial, antifungal, antiviral, and antioxidant activities [13]. Ziziphus also known as “Sedra”s is an important genus of the family Rhamnaceae found growing extensively in arid and semi-arid regions and represented byy135–170 species [14]. It was reported that the fungal crude extract of Trichoderma viride isolated from the medicinal plant of Ziziphus mauritiana was displayed anticancer activity against HeLa cell line [15]. Leaf extract of Z. mauritiana has potential antimicrobial against B. cereus, S. aureus, S. pneumoniae, B. subtilis, P. vulgaris, E. coli, and C. albicans [16]. Although Ziziphus spina-christi (Z. spina-christi) is common in the environment, few studies studied their associated endophytic fungi [17]. Herein, this study is conducted to isolate fungal endophytes from medicinal plant Z. spina-christi and evaluate their antimicrobial, antioxidant, as well as cytotoxicity activities.
Materials and Methods
Collection of Plant Materials
Disease free and mature leaves of Z. spina-christi were collected from Menofia Governorate. Fresh leaf samples were transported to the lab under aseptic conditions for further isolation of endophytic fungi.
Test Microorganisms
Pathogenic microorganisms used in this study were kindly provided from the culture collection unit at the Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University against Gram-negative bacteria (E. coli ATCC 11229, P. vulgaris RCMB 004, P. aeruginosa, and K. pneumonia RCMB 003), Gram-positive bacteria (B. subtilis RCMB 015, S. aureus ATCC 25923, and S. epidermidis ATCC 14990), and unicellular fungi (C. albicans ATCC 90028.
Isolation of Endophytic Fungi
Isolation of endophytic fungi was performed after removing epiphytes from leaf surface with water according to the method described by Strobel and Daisy [18] with slight modification. Firstly, fresh leaves were washed thoroughly in running tap water for 10 min and sterilized in series with 70% ethanol forr1 min and 1.0% sodium hypochlorite (NaOCl) (v/v) for 1 min and further cleaned by passing through two sets of sterile distilled water. After sterilization, leaves were cut into small pieces, 1 cm long, and placed on a plate containing potato dextrose agar (PDA) medium amended with 250 µg/mL streptomycin to suppress bacterial contamination. The plates were incubated at 28 °C until the fungal mycelial started growing on the samples [19, 20]. The last wash water was spread onto PDA plates and served as a negative control to evaluate the success of sterilization [21]. Hyphal tips of emerging colonies from the cultivated leaf sections were sub-cultured on fresh PDA plates to get its pure culture [22].
Morphological and Molecular Identification of Fungal Endophytes
The isolated fungal endophyte was identified morphologically according to Khalil et al. [6]. Macroscopic morphological features including color, texture, and diameter of colonies and microscopic characteristics including vegetative and reproductive structures of the fungus were observed [23,24,25,26,27,28]. The genomic DNA was isolated and purified using Quick-DNA Fungal Microprep Kit (Zymo research; D6007), and molecular identification was achieved by internal transcribed spacer (ITS) region. Gene JET PCR Purification Kit (Thermo K0701) was used for purification of PCR product according to the manufacturer’s protocol. The resulting PCR products were sequenced by sequencing ready reaction kit (Applied Biosystems, Foster, CA, USA). Similar sequences via BLAST search database in the NCBI were compared with product sequence. Evolutionary study was directed in molecular evolutionary genetics analysis MEGA-x software [29,30,31].
Extraction of Bioactive Secondary Metabolites
The bioactive secondary metabolites were extracted following the protocol suggested by Kjer et al. [22].Concretely, 2–3 mycelial plugs were removed from the actively growing edge of the pure fungal colony and inoculated in 1000 mL liquid Wickerham’s medium (0.3% yeast extract, 0.3% malt extract, 0.5% peptone, 1% glucose). Cultures were incubated at 28 °C in static and dark conditions for 21 days. After incubation period, the fermented broth was filtered through filter paper, and the metabolites produced by the fungus were extracted by equal volume of ethyl acetate and hexane separately. An equal volume of ethyl acetate and hexane separately was added to the filtrate and vigorously shaken for 5 min. The mixtures were transferred to separating funnels, and the organic layers of ethyl acetate and hexane were allowed to separate from the aqueous layers. Then, the ethyl acetate and hexane layers were allowed to dry at room temperature. The dried extracts were stored at 4 °C for further use.
Phytochemical Screening of EA Extract
Qualitative screening of many phytochemicals (alkaloids, tannins, flavonoids, saponins, glycosides, phenols, steroids, and terpenoids) was evaluated according to Sarkar et al. [32], Kumar et al. [33], Onwukaeme et al. [34], Auwal et al. [35], and Raaman [36].
Gas Chromatography-Mass Spectrometry (GC–MS) Analysis
EA crude extract of A. alternaria was injected to GC–MS to identify the metabolic compounds. GC–MS analysis was achieved using Agilent Technologies GC–MS 5977A operating at 70 eV and computer mass spectral library (NIST, 2011 version). The spectrum of the unknown constituents was matching with the available data stored in GC–MS libraries.
In Vitro Assessment of Antimicrobial Activity of Alternaria alternata Extracts
The antimicrobial efficacy of A. alternate crude extracts was assessed against different human pathogenic microorganisms as against Gram-negative bacteria (E. coli, P. vulgaris, P. aeruginosa, and K. pneumonia), Gram-positive bacteria (B. subtilis, S. aureus, and S. epidermidis), and unicellular fungi (C. albicans). The methodology was performed using agar well diffusion assay as described by Gauchan et al. [37]. The dried extracts of the fungal strain were dissolved in dimethyl sulphoxide (DMSO). The microbial cultures were spread over the surface of sterilized nutrient agar and yeast extract peptone dextrose agar plates using sterile cotton swab. Wells of 6-mm diameter was made on the plates using a sterile borer. One hundred microliters of 1 mg/mL of fungal extracts dissolved in DMSO was added to the well. DMSO was used as negative control, and gentamycin (10 µg/mL) and fluconazole (20 mg/mL) were used as positive control. The plates were incubated at 37 °C for 24 h. The zone of inhibition was observed and measured. The inhibition zones with a diameter of less than 12 mm were considered having no antibacterial activity [38].
Transmission Electron Microscopy (TEM)
In order to study the effect of EA crude extract of A. alternata on ultrastructure of the most sensitive bacteria, bacterial cells were collected by centrifugation at 4000 rpm for 10 min from 24-h old cultures grown on nutrient broth media and washed with distilled water; the samples were fixed in 3% glutaraldehyde, rinsed in phosphate buffer, and post-fixed in potassium permanganate solution for 5 min at room temperature. The samples were dehydrated in an ethanol series ranging from 10 to 90% for 15 min in each alcohol dilution and finally with absolute ethanol for 30 min. Samples were infiltrated with epoxy resin and acetone through a graded series until finally in pure resin. Ultrathin sections were collected on copper grids. Sections were then double stained in uranyl acetate followed by lead citrate. Stained sections were observed with a JEOL—JEM 1010 TEM at 80 kV at RCMB, Al-Azhar University [39, 40].
Antioxidant Activity
Antioxidant activity of EA crude extract of A. alternata at various concentrations was carried out using DPPHH (2, 2-diphenyl-1Ppicrylhydrazyl) method by Khalil et al. [6] with minor modifications. Different concentrations of crude extracts (2000, 1000, 500, 250, 125, 62.5, 31.25, 15.62, and 7.81 µg/mL) were used to determine the scavenging of DPPH radicals. Antioxidant activity of standard and extracts was determined as DPPH scavenging activity (%): [((control absorbance – extract absorbance) / (control absorbance)) × 100] [41].
In Vitro Cytotoxicity Assay
The cytotoxicity of EA crude extract of A. alternata at concentrations 4000—250 µg/mL was determined using the MTT protocol [42] with minor modification against normal Vero cell lines which collected from ATCC. The viability and inhibition percentages were calculated as shown in Eqs. 1 and 2 as follows:
Statistical Analysis
The data were expressed as the mean ± SDEV value, which was calculated by using Minitab 18 software extended with a statistical package and Microsoft Excel 365.
Results and Discussion
Isolation and Identification of Endophytic Fungus
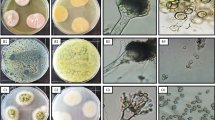
In this study, leaves of Z. spina-christi were used for isolation of fungal endophytes. During sterilization process, there was no mycelium growth on the control plates, indicating the success of sterilization procedure [43]. One fungal isolate was isolated from Z. spina-christi leaves; this strain was completely defined through traditional and molecular identification. Morphological identification revealed that diameter was 75–80 mm after 7 days, color is umber to olivaceous on PDA, and conidia comprising 1–7 transverse septa were narrow-ellipsoid or ovoid as shown in Fig. 1 A and B. The present fungus was morphologically similar to Alternaria alternata. Molecular identification confirmed that this strain is similar to A. alternata with 99% and recorded in gene bank with accession number OM331682. The phylogenetic analysis of fungal strains revealed 98% identity with ITS sequences of rRNA genes of related species using BLAST programs. Ascomycota and Deuteromycota are common for living fungal endophytes [44, 45]. Fungal endophytes Alternaria spp. were isolated in recent studies such as A. alternata [46,47,48] and A. tenuissima [6, 49].
Phytochemical Analyses
Fungal endophytes are well known to produce large amount of novel antimicrobial and antioxidant compounds [50]. In the current study, EA extract contains on alkaloids, tannins, flavonoids, glycosides, phenols, and terpenoids as shown in Table 1. Flavonoids often inhibit fungal growth with various underlying mechanisms, including plasma membrane disruption, the induction of mitochondrial dysfunction, and inhibition of the following: cell wall formation, cell division, RNA and protein synthesis, and the efflux-mediated pumping system [51]. Glycosides serve as antifungal agent through acts as a specific inhibitor of glucan synthesis in cells and in vitro and lead to morphological changes in yeasts and molds [52]. Additionally, the antimicrobial activity may be referred to the presence of tannins because of its ability in molecular inhibitions of the cell membrane of microorganisms, where it decreases the development of complexes that keep its integrity, producing distortions and increasing their penetrability. Also, it affects extracellular microbial enzymes that lead to decrease in the essential compounds for cell development [53]. Phenolic compounds are a group of secondary metabolites holding functional hydroxyl group (-OH) linkage to aromatic hydrocarbon ring which are imperative bioactive compounds because their hydroxyl groups confer scavenging ability [54]. Terpenoids possess antitumor, anti-inflammatory, antibacterial, antiviral, and antimalarial effects, promote transdermal absorption, prevent and treat cardiovascular diseases, and have hypoglycemic activities [55].
GC–MS Analysis of Bioactive Compounds
Gas chromatograph attached with mass spectrometer is one of the most accepted methods that is used for analyzing phytochemical compounds of natural origin because of their stability, sensitivity, and high efficiency [56]. Endophytic fungi living in medicinal plants can make the same pharmacological bioactive secondary metabolites in the same way as their host medicinal plants, which have been used for a long time in traditional medicine and even now are utilized for their health advantages [57, 58]. Results of GC–MS analysis of EA extract of A. alternata are illustrated in Table 2. Results revealed that EA extract of A. alternata contains 16 different compounds, where major compounds were oleic acid methyl ester and linolelaidic acid methyl ester with ratios 41.55 and 13.92%, respectively. Oleic acid is used as anti-inflammatory, anti-cancer, allergenic and insecticide properties, antioxidant, antimicrobial activities, cancer enzyme inhibitors [59, 60] [61]. On the other hand, minor compounds were hexadecanoic acid methyl ester, stearic acid methyl ester, cis-5,8,11,14,17-eicosapentaenoic acid, 9,12-octadecadienoic acid (Z,Z), 6,9,12-octadecatrienoic acid, methyl ester, cis-13-eicosenoic acid, methyl ester, eicosanoic acid, methyl ester, erucic acid, behenic acid, methyl ester, 1,2-benzenedicarboxylic acid (diisooctyl ester), tetracosanoic acid, methyl ester, linoleic acid ethyl ester, hexacosanoic acid, methyl ester, and stigmastan-3,5-diene with ratios 3.11, 5.81, 2.13, 0.95, 1.09, 3.20, 2.99, 3.48, 8.85, 2.17, 7.43, 0.36, 0.52, and 0.46%, respectively. These compounds have different biological activities such as antimicrobial, antioxidant, hypocholesterolemic, nematicide, pesticide, antiandrogenic, anticolorectal cancer activity, hepatoprotective, antihistamine, hypocholesterolemic, anti-eczemic, antistaphylococcal, antihypertensive, and antiulcer activities as illustrated in Table 2 and Fig. 2.
Antimicrobial Activity
The EA and hexane crude extracts obtained from A. alternata were evaluated for their antimicrobial activity against human pathogenic microorganisms by agar well diffusion method. Data presented in Table 3 clearly indicated that both crude extracts exhibited different degree of inhibition as compared to controls. Interestingly, ethyl acetate displayed a strong inhibitory activity against all Gram-negative and Gram-positive bacteria and unicellular fungi, whereas the hexane crude extract exhibited weak growth inhibition against tested organisms and did not affect the growth of P. vulgaris. The maximum inhibitory activity of the EA crude extract was recorded against K. pneumonia with inhibition zone of 49 ± 0.05 mm followed by S. epidermidis 43 ± 0.00 mm, E. coli 42 ± 0.2 mm, P. vulgaris 41 ± 0.08 mm, and B. subtilis 40.06 ± 0.1 mm as shown in Fig. 3. These results demonstrated that EA crude extract presented high broad-spectrum activity as compared to hexane extract. That means ethyl acetate contains the maximum number/concentration of bioactive compounds which directly or indirectly influences the inhibition zone. Activity of secondary metabolites is attributed to tier ability to cell wall synthesis and depolarizes the cell membrane, inhibition of protein synthesis, inhibition of nucleic acid synthesis, and metabolic pathways inhibition in bacteria [72, 73]. Previous reports evaluated that the activity of the prepared extracts from the leaves and fruits depends on the active ingredients as well as the polarity of the ingredients [74, 75]. In addition, several studies showed that the extracts of different polarity give potentially different pharmacological and toxicological activities [76, 77]. Our results are in accordance to Al Mousa et al. [78] who stated that the EA crude extract obtained from Alternaria tenuissima AUMC14342 gave the highest antimicrobial activity against P. aeruginosa, S. aureus, Fusarium solani, and Aspergillus niger at concentration 30 mg/mL using disc diffusion assay. Moreover, our results are in agreement with Techaoei et al. [79] who found that the EA crude extract of A. alternata isolated from lotus displayed more potential against both S. epidermidis and Methicillin-resistant Staphylococcus aureus (MRSA). Similarly, Tang et al. [80] reported that the EA crude extract of Penicillium oxalicum had antibacterial effects against all the tested bacteria with MIC between 0.50 and 2 mg/mL. Also, only the EA extract of Simplicillium sp. showed antibacterial effect against E. coli, P. aeruginosa, B. subtilis, and S. aureus with MIC of 0.5, 1, 2, and 1 mg/mL, respectively. Chatterjee et al. [81] revealed that the EA extract of endophytic fungus A. alternata VN3 isolated from Vitex negundo L. was also effective against both Gram-positive and Gram-negative bacteria.
Ultrastructure Study
To confirm antibacterial activity of EA extract A. alternata, ultrastructure of treated K. pneumonia by this extract was carried out as shown in Fig. 4. Rod-shaped cells, smooth continuous cell wall and cell membrane, homogeneous electron dense cytoplasm, and normal electron lucent zone between cell wall and cell membrane are seen in this transmission electron micrograph of typical K. pneumonia (Fig. 4A). On the other hand, treated K. pneumonia with EA crude extract of A. alternata displayed deformed cells with rough uneven cell walls appear to be seeping from the damaged membrane with electron lucent patches emerge in the cytoplasm; remarkably elucidated destruction of the cell wall and cell membrane. These treated cells obviously revealed leakage of cytoplasmic materials, concerned in the center of the cell leave large space in between as shown in Fig. 4B. Antimicrobial agents affect bacterial cell membrane leading to complete damage of the cells [82]. Nath and Joshi [83] studied the effect of ethanolic extract of endophytic fungus Glomerella acutata EF15 on K. pneumonia and found that bacterial cells appeared crumpled and shrunken, and cavity formation was prominent on the cell membrane of the bacteria which lead to complete damage.
Antioxidant Activity
This work was designed to obtain fungal endophytes with promising antioxidant activities from Z. spina-christi plants. Biological reactions usually produce reactive oxygen species (ROS) as by-products which causes cell death due to oxidative damage to biological materials [84]. Beating the harmful effect of ROS in human organs, external source of antioxidant should be useful. However, one of the main properties of antioxidant molecules is their capability to hold and balance free radicals [85]. Antioxidants have been considered therapy agents where they possess anti-atherosclerotic, anti-inflammatory, antitumor, anticarcinogenic, antimutagenic, and antimicrobial properties. Antioxidants are frequently found naturally in medicinal herbs, vegetables, and fruits. In our study, EA crude extract of A. alternata was assessed as antioxidant using DPPH method as shown in Fig. 5. Results revealed that EA crude extract of A. alternata exhibited promising antioxidant activity as compared to ascorbic acid, where activity at 500–2000 µg/mL was above 50%. Also, results illustrated that IC50 of EA crude extract of A. alternata was 409 µg/mL. This activity is attributed to presence phenolic compounds which are confirmed by phytochemical screening and GC–MS. Ibrahim et al. [86] reported that EA crude extract of Alternaria sp. showed potential antioxidant activity and IC50 was 520 µg/mL. Moreover, Khiralla et al. [87] isolated Alternaria sp. from leaves of Calotropis procera, where it exhibited antioxidant activity with MIC 236 µg/mL. Another study confirmed that EA extract of Alternaria sp. (ML4) had DPPH scavenging activity of 85.20% at the concentration of 300 µg/mL and high reducing power activity [88].
Cytotoxic Activity
Evaluation of cytotoxicity of the natural metabolic products is the first step to determine their safety on noncancerous human cells [89]. Cytotoxicity of EA crude extract of A. alternata was determined toward Vero normal cell line as illustrated in Fig. 6. Results revealed that concentration of EA crude extract of A. alternata at < = 1000 µg/ml did not show any toxicity on Vero cell line. Also, IC50 was greater than 4000 µg/mL, where if the IC50 is ≥ 90 µg/mL, the compound is classified as not cytotoxic [90]. Eventually, EA crude extract of A. alternata is non-toxic and safe for use.
Conclusion
In the current study, promising endophytic A. alternata was isolated from leaves of Z. spina-christi and deposited in gene bank with accession number OM331682. Bioactive compounds which were produced by endophytic A. alternata were analyzed and determined through GC–MS and phytochemical analyses. Crude extract of A. alternata has promising antibacterial and antifungal properties against Gram-negative, Gram-positive, and unicellular fungi. Moreover, ultrastructure study confirmed damaging cell wall and cell membrane and leakage of cytoplasmic materials. Furthermore, this extract has potential antioxidant activity as well as no toxicity on normal cell line. Eventually, the crude extract of endophytic A. alternata is recommended as bioactive compounds for different biological applications.
References
Elbasuney, S., El-Sayyad, G. S., Tantawy, H., & Hashem, A. H. (2021). Promising antimicrobial and antibiofilm activities of reduced graphene oxide-metal oxide (RGO-NiO, RGO-AgO, and RGO-ZnO) nanocomposites. RSC Advances, 11(42), 25961–25975. https://doi.org/10.1039/D1RA04542C.
Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD (2010) Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: Systematic review and meta-analysis. Bmj 340
Sharaf MH, Abdelaziz AM, Kalaba MH, Radwan AA, Hashem AH (2021) Antimicrobial, antioxidant, cytotoxic activities and phytochemical analysis of fungal endophytes isolated from ocimum basilicum. Applied biochemistry and biotechnology:1–19
Murthy, N. K., Pushpalatha, K., & Joshi, C. G. (2011). Antioxidant activity and phytochemical analysis of endophytic fungi isolated from Lobelia nicotianifolia. Journal of Chemical and Pharmaceutical Research, 3(5), 218–225.
Meena, K. K., Sorty, A. M., Bitla, U. M., Choudhary, K., Gupta, P., Pareek, A., et al. (2017). Abiotic stress responses and microbe-mediated mitigation in plants: The omics strategies. Frontiers in Plant Science, 8, 172.
Khalil, A., Abdelaziz, A., Khaleil, M., & Hashem, A. (2021). Fungal endophytes from leaves of Avicennia marina growing in semi-arid environment as a promising source for bioactive compounds. Letters in Applied Microbiology, 72(3), 263–274.
Aly, A. H., Debbab, A., & Proksch, P. (2011). Fungal endophytes: Unique plant inhabitants with great promises. Applied microbiology and biotechnology, 90(6), 1829–1845.
Ancheeva, E., Daletos, G., & Proksch, P. (2020). Bioactive secondary metabolites from endophytic fungi. Current medicinal chemistry, 27(11), 1836–1854.
Kouipou Toghueo, R. M., & Boyom, F. F. (2019). Endophytic fungi from Terminalia species: A comprehensive review. Journal of Fungi, 5(2), 43.
Brase, S., Encinas, A., Keck, J., & Nising, C. F. (2009). Chemistry and biology of mycotoxins and related fungal metabolites. Chemical reviews, 109(9), 3903–3990.
Tsuge, T., Harimoto, Y., Akimitsu, K., Ohtani, K., Kodama, M., Akagi, Y., et al. (2013). Host-selective toxins produced by the plant pathogenic fungus Alternaria alternata. FEMS microbiology reviews, 37(1), 44–66.
Huang, W.-Y., Cai, Y.-Z., Xing, J., Corke, H., & Sun, M. (2007). A potential antioxidant resource: Endophytic fungi from medicinal plants. Economic botany, 61(1), 14–30.
Tolulope, R., Adeyemi, A., Erute, M., & Abiodun, T. (2015). Isolation and screening of endophytic fungi from three plants used in traditional medicine in Nigeria for antimicrobial activity. International Journal of Green Pharmacy, 9(1), 58.
Maraghni, M., Gorai, M., & Neffati, M. (2010). Seed germination at different temperatures and water stress levels, and seedling emergence from different depths of Ziziphus lotus. South African Journal of Botany, 76(3), 453–459.
Sheeba, H., Ali, M., & Anuradha, V. (2020). In-vitro anti-cancer activity of endophytic fungi isolated from Ziziphus mauritiana in cervical cancer cell line. Eur J Med Plants, 31, 38–48.
Abdallah EM, Elsharkawy ER, Ed-dra A (2016) Biological activities of methanolic leaf extract of Ziziphus mauritiana. Pharm Commun Biosci Biotech Res Comm Thomson Reuters ISI ESC Crossref Index J NAAS J Score 9 (4):605–614
EL-NAGERABI SA, Elshafie AE, Alkhanjari SS (2013) Endophytic fungi associated with Ziziphus species and new records from mountainous area of Oman. Biodiversitas Journal of Biological Diversity 14 (1)
Strobel, G., & Daisy, B. (2003). Bioprospecting for microbial endophytes and their natural products. Microbiology and Molecular Biology Reviews, 67(4), 491–502. https://doi.org/10.1128/MMBR.67.4.491-502.2003.
Sharaf, M. H., Abdelaziz, A. M., Kalaba, M. H., Radwan, A. A., & Hashem, A. H. (2022). Antimicrobial, antioxidant, cytotoxic activities and phytochemical analysis of fungal endophytes isolated from ocimum basilicum. Applied biochemistry and biotechnology, 194(3), 1271–1289. https://doi.org/10.1007/s12010-021-03702-w.
Badawy, A. A., Alotaibi, M. O., Abdelaziz, A. M., Osman, M. S., Khalil, A. M. A., Saleh, A. M., et al. (2021). Enhancement of seawater stress tolerance in barley by the endophytic fungus Aspergillus ochraceus. Metabolites, 11(7), 428.
Maliehe, T. S., Mbambo, M., Nqotheni, M. I., Senzo, N. S., & Shandu, J. S. E. (2022). Antibacterial effect and mode of action of secondary metabolites from fungal endophyte associated with Aloe ferox Mill. Microbiology Research, 13(1), 90–101.
Kjer, J., Debbab, A., Aly, A. H., & Proksch, P. (2010). Methods for isolation of marine-derived endophytic fungi and their bioactive secondary products. Nature protocols, 5(3), 479–490.
Hashem, A. H., Hasanin, M. S., Khalil, A. M. A., & Suleiman, W. B. (2020). Eco-green conversion of watermelon peels to single cell oils using a unique oleaginous fungus: Lichtheimia corymbifera AH13. Waste and Biomass Valorization, 11(11), 5721–5732. https://doi.org/10.1007/s12649-019-00850-3.
Hashem, A. H., Al Abboud, M. A., Alawlaqi, M. M., Abdelghany, T. M., & Hasanin, M. (2022). Synthesis of nanocapsules based on biosynthesized nickel nanoparticles and potato starch: Antimicrobial, antioxidant, and anticancer activity. Starch-Stärke, 74(1–2), 2100165.
Hashem, A. H., Abu-Elreesh, G., El-Sheikh, H. H., & Suleiman, W. B. (2022). Isolation, identification, and statistical optimization of a psychrotolerant Mucor racemosus for sustainable lipid production. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-022-02390-8.
Hashem AH, Al Abboud MA, Alawlaqi MM, Abdelghany TM, Hasanin M (2022) Synthesis of nanocapsules based on biosynthesized nickel nanoparticles and potato starch: Antimicrobial, antioxidant, and anticancer activity.
Hasanin, M., Hashem, A. H., Lashin, I., & Hassan, S. A. M. (2021). In vitro improvement and rooting of banana plantlets using antifungal nanocomposite based on myco-synthesized copper oxide nanoparticles and starch. Biomass Conversion and Biorefinery. https://doi.org/10.1007/s13399-021-01784-4.
Hasanin, M., Al Abboud, M. A., Alawlaqi, M. M., Abdelghany, T. M., & Hashem, A. H. (2021). Ecofriendly synthesis of biosynthesized copper nanoparticles with starch-based nanocomposite: Antimicrobial, antioxidant, and anticancer activities. Biological Trace Element Research. https://doi.org/10.1007/s12011-021-02812-0.
MEGA X molecular evolutionary genetics analysis across computing platforms; S Kumar, G Stecher, M Li, C Knyaz, K Tamura. Molecular Biology and Evolution:1547–1549
Khalil, A. M. A., Hashem, A. H., & Abdelaziz, A. M. (2019). Occurrence of toxigenic Penicillium polonicum in retail green table olives from the Saudi Arabia market. Biocatalysis and Agricultural Biotechnology, 21,. https://doi.org/10.1016/j.bcab.2019.101314.
Ahmed, N. E., Salem, S. S., & Hashem, A. H. (2021). Statistical optimization, partial purification, and characterization of phytase produced from Talaromyces purpureogenus NSA20 using potato peel waste and its application in dyes de-colorization. Biointerface Research in Applied Chemistry, 12(4), 4417–4431.
Sarkar, T., Salauddin, M., Pati, S., Sheikh, H. I., & Chakraborty, R. (2021). Application of raw and differently dried Pineapple (Ananas comosus) pulp on Rasgulla (sweetened Casein Ball) to enhance its phenolic profile, shelf life, and in-vitro digestibility characteristics. Journal of Food Processing and Preservation, 45(3).
Kumar RS, Balasubramanian P, Govindaraj P, Krishnaveni T (2014) Preliminary studies on phytochemicals and antimicrobial activity of solvent extracts of Coriandrum sativum L. roots (Coriander). Journal of Pharmacognosy and Phytochemistry 2 (6)
Onwukaeme, D., Ikuegbvweha, T., & Asonye, C. (2007). Evaluation of phytochemical constituents, antibacterial activities and effect of exudate of Pycanthus Angolensis Weld Warb (Myristicaceae) on corneal ulcers in rabbits. Tropical Journal of Pharmaceutical Research, 6(2), 725–730.
Auwal MS, Saka S, Mairiga IA, Sanda KA, Shuaibu A, Ibrahim A Preliminary phytochemical and elemental analysis of aqueous and fractionated pod extracts of Acacia nilotica (Thorn mimosa). In: Veterinary research forum: an international quarterly journal, 2014. vol 2. Faculty of Veterinary Medicine, Urmia University, Urmia, Iran, p 95
Raaman N (2006) Phytochemical techniques (pp. 19–22). New Delhi: New India Publishing Agency, Jai Bharat Printing Press,
Gauchan DP, Kandel P, Tuladhar A, Acharya A, Kadel U, Baral A, Shahi AB, García-Gil MR (2020) Evaluation of antimicrobial, antioxidant and cytotoxic properties of bioactive compounds produced from endophytic fungi of Himalayan yew (Taxus wallichiana) in Nepal. F1000Research 9
Durairaj, S., Srinivasan, S., & Lakshmanaperumalsamy, P. (2009). In vitro antibacterial activity and stability of garlic extract at different pH and temperature. Electronic Journal of Biology, 5(1), 5–10.
Amin, B. (2016). Isolation and characterization of antiprotozoal and antimicrobial metabolite from Penicillium roqueforti. Afr J Mycol Biotech, 21, 13–26.
Amin, B. H., Abou-Dobara, M. I., Diab, M. A., Gomaa, E. A., El-Mogazy, M. A., El-Sonbati, A. Z., & EL-Ghareib MS, Hussien MA, Salama HM,. (2020). Synthesis, characterization, and biological investigation of new mixed-ligand complexes. Applied Organometallic Chemistry, 34(8).
Hashem, A. H., Khalil, A. M. A., Reyad, A. M., & Salem, S. S. (2021). Biomedical applications of mycosynthesized selenium nanoparticles using Penicillium expansum ATTC 36200. Biological Trace Element Research, 199(10), 3998–4008. https://doi.org/10.1007/s12011-020-02506-z.
Van de Loosdrecht, A., Beelen, R., & Ossenkoppele g, Broekhoven M, Langenhuijsen M,. (1994). A tetrazolium-based colorimetric MTT assay to quantitate human monocyte mediated cytotoxicity against leukemic cells from cell lines and patients with acute myeloid leukemia. Journal of Immunological Methods, 174(1–2), 311–320.
Almaary KS, Alharbi NS, Kadaikunnan S, Khaled JM, Rajivgandhi G, Ramachandran G, Kanisha CC, Murugan M, Alanzi KF, Manoharan N (2021) Anti-bacterial effect of marine sea grasses mediated endophytic actinomycetes against K. pneumoniae. Journal of King Saud University-Science 33 (6):101528
Petrini, O., & Müller, E. (1979). Pilzliche Endophyten, am Beispiel von Juniperus communis L. Sydowia, 32, 224–251.
Bharathidasan, R., & Panneerselvam, A. (2011). Isolation and identification of endophytic fungi from Avicennia marina in Ramanathapuram District, Karankadu, Tamilnadu. India. European Journal of Experimental Biology, 1(3), 31–36.
Chandra, H., Kumari, P., Prasad, R., Gupta, S. C., & Yadav, S. (2021). Antioxidant and antimicrobial activity displayed by a fungal endophyte Alternaria alternata isolated from Picrorhiza kurroa from Garhwal Himalayas. India. Biocatalysis and Agricultural Biotechnology, 33,.
Lee, C., Li, W., Bang, S., Lee, S. J., Kang, N.-Y., Kim, S., et al. (2019). Secondary metabolites of the endophytic fungus Alternaria alternata JS0515 isolated from Vitex rotundifolia and their effects on pyruvate dehydrogenase activity. Molecules, 24(24), 4450. https://doi.org/10.3390/molecules24244450.
Khiralla A, Mohamed IE, Tzanova T, Schohn H, Slezack-Deschaumes S, Hehn A, André P, Carre G, Spina R, Lobstein A (2016) Endophytic fungi associated with Sudanese medicinal plants show cytotoxic and antibiotic potential. FEMS microbiology letters 363 (11)
Lindblom, S. D., Wangeline, A. L., Valdez Barillas, J. R., Devilbiss, B., Fakra, S. C., & Pilon-Smits, E. A. H. (2018). Fungal endophyte Alternaria tenuissima can affect growth and selenium accumulation in its hyperaccumulator host Astragalus bisulcatus. Frontiers in Plant Science. https://doi.org/10.3389/fpls.2018.01213.
Shukla, S., Habbu, P., Kulkarni, V., Jagadish, K., Pandey, A., & Sutariya, V. (2014). Endophytic microbes: A novel source for biologically/pharmacologically active secondary metabolites. Asian J Pharmacol Toxicol, 2(3), 1–6.
Al Aboody, M. S., & Mickymaray, S. (2020). Anti-fungal efficacy and mechanisms of flavonoids. Antibiotics, 9(2), 45.
Schwartz, R. E., Smith, S. K., Onishi, J. C., Meinz, M., Kurtz, M., Giacobbe, R. A., et al. (2000). Isolation and structural determination of enfumafungin, a triterpene glycoside antifungal agent that is a specific inhibitor of glucan synthesis. Journal of the American Chemical Society, 122(20), 4882–4886.
Liu, P., Kumar, I., Brown, S., Kannappan, V., Tawari, P., Tang, J., et al. (2013). Disulfiram targets cancer stem-like cells and reverses resistance and cross-resistance in acquired paclitaxel-resistant triple-negative breast cancer cells. British journal of cancer, 109(7), 1876–1885.
Mohamed, M. S., Saleh, A. M., Abdel-Farid, I. B., & El-Naggar, S. A. (2017). Growth, hydrolases and ultrastructure of Fusarium oxysporum as affected by phenolic rich extracts from several xerophytic plants. Pesticide biochemistry and physiology, 141, 57–64.
Yang W, Chen X, Li Y, Guo S, Wang Z, Yu X (2020) Advances in pharmacological activities of terpenoids. Natural Product Communications 15 (3):1934578X20903555
Guo, L., Xie, M.-Y., Yan, A.-P., Wan, Y.-Q., & Wu, Y.-M. (2006). Simultaneous determination of five synthetic antioxidants in edible vegetable oil by GC–MS. Analytical and bioanalytical chemistry, 386(6), 1881–1887.
Kaul, S., Gupta, S., Ahmed, M., & Dhar, M. K. (2012). Endophytic fungi from medicinal plants: A treasure hunt for bioactive metabolites. Phytochemistry reviews, 11(4), 487–505.
Abo Nahas, H. H. (2019). Endophytic fungi: A gold mine of antioxidants. Microbial Biosystems, 4(1), 58–79.
Prabhadevi V, Sahaya SS, Johnson M, Venkatramani B, Janakiraman N (2012) Phytochemical studies on Allamanda cathartica L. using GC–MS. Asian Pacific Journal of Tropical Biomedicine 2 (2):S550-S554
El-Fayoumy, E. A., Shanab, S. M., Gaballa, H. S., Tantawy, M. A., & Shalaby, E. A. (2021). Evaluation of antioxidant and anticancer activity of crude extract and different fractions of Chlorella vulgaris axenic culture grown under various concentrations of copper ions. BMC Complementary Medicine and Therapies, 21(1), 1–16.
Dilika, F., Bremner, P. D., & Meyer, J. J. (2000). Antibacterial activity of linoleic and oleic acids isolated from Helichrysum pedunculatum: A plant used during circumcision rites. Fitoterapia, 71(4), 450–452. https://doi.org/10.1016/s0367-326x(00)00150-7.
Anbazhagi T, Kadavul K, Suguna G, Petrus A (2009) Studies on the pharmacognostical and in vitro antioxidant potential of Cleome gynandra Linn. leaves.
da Silva, L. L. D., Nascimento, M., Silva, D. H. S., Furlan, M., & da Silva, B. V. (2002). Antibacterial activity of a stearic acid derivative from Stemodia foliosa. Planta medica, 68(12), 1137–1139.
Shin, S. Y., Bajpai, V. K., Kim, H. R., & Kang, S. C. (2007). Antibacterial activity of eicosapentaenoic acid (EPA) against foodborne and food spoilage microorganisms. LWT - Food Science and Technology, 40(9), 1515–1519. https://doi.org/10.1016/j.lwt.2006.12.005.
Cockbain, A. J., Volpato, M., Race, A. D., Munarini, A., Fazio, C., Belluzzi, A., et al. (2014). Anticolorectal cancer activity of the omega-3 polyunsaturated fatty acid eicosapentaenoic acid. Gut, 63(11), 1760–1768.
Chenniappan J, Sankaranarayanan A, Arjunan S (2020) Evaluation of antimicrobial activity of Cissus quadrangularis L. stem extracts against avian pathogens and determination of its bioactive constituents using GC-MS. Journal of scientific research 64 (1)
Gulfraz, M., Sadiq, A., Tariq, H., Imran, M., Qureshi, R., & Zeenat, A. (2011). Phytochemical analysis and antibacterial activity of Eruca sativa seed. Pakistan Journal of Botany, 43(2), 1351–1359.
Rajeswari G, Murugan M, Mohan V (2012) GC-MS analysis of bioactive components of Hugonia mystax L.(Linaceae). Research Journal of Pharmaceutical, Biological and chemical sciences 3 (4):301–308
Rahman, M., & Anwar, M. (2006). Fungitoxic and cytotoxic activity of a novel compound 1, 2-benzenedicarboxylic acid, diisooctyl ester of Plumbago zeylanica linn. ASIAN JOURNAL OF MICROBIOLOGY BIOTECHNOLOGY AND ENVIRONMENTAL SCIENCES, 8(3), 461.
Li, M., Zhou, L., Yang, D., Li, T., & Li, W. (2012). Biochemical composition and antioxidant capacity of extracts from Podophyllum hexandrum rhizome. BMC complementary and alternative medicine, 12(1), 1–8.
Altameme, H. J., Hameed, I. H., & Abu-Serag, N. A. (2015). Analysis of bioactive phytochemical compounds of two medicinal plants, Equisetum arvense and Alchemila valgaris seed using gas chromatographymass spectrometry and fourier-transform infrared spectroscopy. Malaysian Applied Biology, 44(4), 47–58.
Ebrahimi A, Asgharian S, Habibian S (2010) Antimicrobial activities of isolated endophytes from some Iranian native medicinal plants.
Reygaert, W. C. (2018). An overview of the antimicrobial resistance mechanisms of bacteria. AIMS microbiology, 4(3), 482.
Al-Saeedi, A. H., Al-Ghafri, M. T. H., & Hossain, M. A. (2017). Brine shrimp toxicity of various polarities leaves and fruits crude fractions of Ziziphus jujuba native to Oman and their antimicrobial potency. Sustainable Chemistry and Pharmacy, 5, 122–126.
Brahmi, F., Mechri, B., Dabbou, S., Dhibi, M., & Hammami, M. (2012). The efficacy of phenolics compounds with different polarities as antioxidants from olive leaves depending on seasonal variations. Industrial Crops and Products, 38, 146–152. https://doi.org/10.1016/j.indcrop.2012.01.023.
Parveen, S., Irfan Bukhari, N., Shehzadi, N., Qamar, S., Ali, E., Naheed, S., et al. (2019). Chemical and pharmacological comparison of modern and traditional dosage forms of Joshanda. Natural product research, 33(6), 858–861.
Hossain, M. A. (2019). A phytopharmacological review on the Omani medicinal plant: Ziziphus jujube. Journal of King Saud University-Science, 31(4), 1352–1357.
Al Mousa AA, Mohamed H, Hassane AM, Abo-Dahab NF (2021) Antimicrobial and cytotoxic potential of an endophytic fungus Alternaria tenuissima AUMC14342 isolated from Artemisia judaica L. growing in Saudi Arabia. Journal of King Saud University-Science 33 (5):101462
Techaoei, S., Jarmkom, K., Dumrongphuttidecha, T., & Khobjai, W. (2021). Evaluation of the stability and antibacterial activity of crude extracts of hydro-endophytic fungi. Journal of Advanced Pharmaceutical Technology & Research, 12(1), 61.
Tang, Z., Qin, Y., Chen, W., Zhao, Z., Lin, W., Xiao, Y., et al. (2021). Diversity, chemical constituents, and biological activities of endophytic fungi isolated from Ligusticum chuanxiong Hort. Frontiers in microbiology, 12, 771000–771000.
Chatterjee, S., Ghosh, R., & Mandal, N. C. (2019). Production of bioactive compounds with bactericidal and antioxidant potential by endophytic fungus Alternaria alternata AE1 isolated from Azadirachta indica A. Juss. Plos one, 14(4).
Darah, I., Lim, S., & Nithianantham, K. (2013). Effects of methanol extract of Wedelia chinensis osbeck (asteraceae) leaves against pathogenic bacteria with emphasise on Bacillus cereus. Indian journal of pharmaceutical sciences, 75(5), 533.
Nath, A., & Joshi, S. R. (2015). Ultrastructural effect on mastitis pathogens by extract of endophytic fungi associated with ethnoveterinary plant. Hibiscus sabdariffa L. J Microsc Ultrastruct, 3(1), 38–43. https://doi.org/10.1016/j.jmau.2014.10.001.
Cui J-L, Guo T-T, Ren Z-X, Zhang N-S, Wang M-L (2015) Diversity and antioxidant activity of culturable endophytic fungi from alpine plants of Rhodiola crenulata, R. angusta, and R. sachalinensis. PloS one 10 (3):e0118204
Prakash, D., Upadhyay, G., Pushpangadan, P., & Gupta, C. (2011). Antioxidant and free radical scavenging activities of some fruits. Journal of Complementary and Integrative Medicine, 8(1), 1–16.
Ibrahim AH, Attia EZ, Abdelmohsen UR, Desoukey SY, Elkhayat ES, Fouad MA, Kamel MS (2018) Lipid profiling, phenolic content and antioxidant activity of the endophytic fungus Alternaria sp. isolated from Dracaena plant. Journal of advanced Biomedical and Pharmaceutical Sciences 1 (1):13–16
Khiralla, A., Mohamed, I., Thomas, J., Mignard, B., Spina, R., Yagi, S., & Laurain-Mattar, D. (2015). A pilot study of antioxidant potential of endophytic fungi from some Sudanese medicinal plants. Asian Pacific Journal of Tropical Medicine, 8(9), 701–704. https://doi.org/10.1016/j.apjtm.2015.07.032.
Gunasekaran, S., Sathiavelu, M., & Arunachalam, S. (2017). In vitro antioxidant and antibacterial activity of endophytic fungi isolated from Mussaenda luteola. J Appl Pharm Sci, 7(8), 234–238.
Devi, N. N., Prabakaran, J. J., & Wahab, F. (2012). Phytochemical analysis and enzyme analysis of endophytic fungi from Centella asiatica. Asian Pacific Journal of Tropical Biomedicine, 2(3), S1280–S1284.
Ioset J-R, Brun R, Wenzler T, Kaiser M, Yardley V (2009) Drug screening for kinetoplastids diseases. A Training Manual for Screening in Neglected Diseases
Acknowledgements
The authors express their sincere thanks to the Faculty of Science (Boys), Al-Azhar University, Cairo, Egypt; Microbiology Lab of the Botany and Microbiology Department, Faculty of Science at Benha University, Benha, Egypt; and The Regional Center for Mycology and Biotechnology (RCMB), Al-Azhar University, for providing the necessary research facilities.
Funding
Open access funding provided by The Science, Technology & Innovation Funding Authority (STDF) in cooperation with The Egyptian Knowledge Bank (EKB).
Author information
Authors and Affiliations
Contributions
Contributions of all authors are equal in methodology and writing.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
Not applicable.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Elghaffar, R.Y.A., Amin, B.H., Hashem, A.H. et al. Promising Endophytic Alternaria alternata from Leaves of Ziziphus spina-christi: Phytochemical Analyses, Antimicrobial and Antioxidant Activities. Appl Biochem Biotechnol 194, 3984–4001 (2022). https://doi.org/10.1007/s12010-022-03959-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-022-03959-9