Abstract
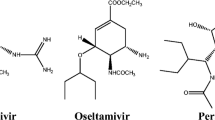
Neuraminidase (NA), a surface protein, facilitates the release of nascent virus and thus spreads infection. It has been renowned as a potential drug target for influenza A virus infection. The drugs such as oseltamivir, zanamivir, peramivir, and laninamivir are approved for the treatment of influenza infection. Additionally, investigational drugs namely MK2206, tamiphosphor, crenatoside, and dehydroepiandrosterone (DHEA) are also available for the treatment. However, recent outbreaks of highly pathogenic and drug-resistant influenza A strains highlighted the need to discover novel NA inhibitor. Keeping this in mind, in the current investigation, an effort was made to ascertain potent inhibitors using pharmacophore-based virtual screening and docking approach. A 3D pharmacophore model was generated based on the chemical features of approved and investigational NA inhibitors using PHASE module of Schrödinger suite. The model consists of two hydrogen bond acceptors (A), one hydrogen bond donor (D), and one positively charged group (P), AADP. Subsequently, molecules with same pharmacophoric features were screened from among the two million compounds available in the ZINC database using the generated pharmacophore hypothesis. Ligand filtration was also done to obtain an efficient collection of hit molecules by employing Lipinski “rule of five” using Qikprop module. Finally, the screened molecule was subjected to docking and molecular dynamic simulations to examine the inhibiting activity of the compounds. The results of our analysis suggest that “acebutolol hydrochloride” (156792) could be the promising candidates for the treatment of influenza A virus infection.








Similar content being viewed by others
References
Li, X. B., Wang, S. Q., Xu, W. R., Wang, R. L., & Chou, K. C. (2011). Novel inhibitor design for hemagglutinin against H1N1 influenza virus by core hopping method. PLoS One, 6, e28111.
Mukhtar, M. M., Rasool, S. T., Song, D., Zhu, C., Hao, Q., & Zhu, Y. (2007). Origin of highly pathogenic H5N1 avian influenza virus in China and genetic characterization of donor and recipient viruses. Journal of General Virology, 88, 3094–3099.
Matrosovich, M. N., Matrosovich, T. Y., Gray, T., Roberts, N. A., & Klenk, H. (2004). Neuraminidase is important for the initiation of influenza virus infection in human airway epithelium. Journal of Virology, 78, 12665–12667.
Su, Y., Yang, H. Y., Zhang, B. J., Jia, H. L., & Tien, P. (2008). Analysis of a point mutation in H5N1 avian influenza virus hemagglutinin in relation to virus entry into live mammalian cells. Archives of Virology, 153, 2253–2261.
Chan, M. C., Cheung, C. Y., Chui, W. H., Tsao, S. W., Nicholls, J. M., Chan, Y. O., Chan, R. W., Long, H. T., Poon, L. L., Guan, Y., & Peiris, J. S. (2005). Pro-inflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respiratory Research, 6, 135.
Bauer, K., Richter, M., Wutzler, P., & Schmidtke, M. (2009). Different neuraminidase inhibitor susceptibilities of human H1N1, H1N2, and H3N2 influenza A viruses isolated in Germany from 2001 to 2005/06. Antiviral Research, 82, 34–41.
Hurt, A. C., Holien, J. K., Parker, M., & Barr, I. G. (2009). Oseltamivir resistance and the H274Y neuraminidase mutation in seasonal, pandemic and highly pathogenic influenza viruses. Drugs, 69, 2523–2531.
Shobugawa, Y., Saito, R., Sato, I., Kawashima, T., Dapat, C., Dapat, I. C., Kondo, H., Suzuki, Y., Saito, K., & Suzuki, H. (2012). Clinical effectiveness of neuraminidase inhibitor—oseltamivir, zanamivir, laninamivir, and peramivir—for treatment of influenza A(H3N2) and A(H1N1) pdm09 infection: an observational study in the 2010-2011 influenza season in Japan. Journal of Infection and Chemotherapy, 18, 858–864.
Pizzorno, A., Abed, Y., Plante, P. L., Carbonneau, J., Baz, M., Hamelin, M. E., Corbeil, J., & Boivin, G. (2014). Evolution of oseltamivir resistance mutations in influenza A(H1N1) and A(H3N2) viruses during selection in experimentally infected mice. Antimicrobial Agents and Chemotherapy, 58, 6398–6405.
Wu, N. C., Young, A. P., Dandekar, S., Wijersuriya, H., Al-Mawsawi, L. Q., Wu, T. T., & Sun, R. (2013). Systematic identification of H274Y compensatory mutations in influenza a virus neuraminidase by high-throughput screening. Journal of Virology, 87, 1193–1199.
Yen, H. L., McKimm-Breschkin, J. L., Choy, K. T., Wong, D. D. Y., Cheung, P. P. H., Zhou, J., Ng, I. H., Zhu, H., Webby, R. J., Guan, Y., Webster, R. G., & Peirisa, J. S. M. (2013). Resistance to neuraminidase inhibitors conferred by an R292K mutation in a human influenza virus H7N9 isolate can be masked by a mixed R/K viral population. MBio, 4, e00396–e00313.
McKimm-Breschkin. (2012). Influenza neuraminidase inhibitors: antiviral action and mechanisms of resistance. Influenza and Other Respiratory Viruses, 7, 25–36.
Escuret, V., Collins, P. J., Casalegno, J. S., Vachieri, S. G., Cattle, N., Ferraris, O., Sabatier, M., Frobert, E., Caro, V., Skehel, J. J., Gamblin, S., Valla, F., Valette, M., Ottmann, M., McCauley, J. W., Daniels, R. S., & Lina, B. (2014). A novel I221L substitution in neuraminidase confers high-level resistance to oseltamivir in influenza B viruses. The Journal of Infectious Diseases, 210, 1260–1269.
LeGoff, J., Rousset, D., Abou-Jaoudé, G., Scemla, A., Ribaud, P., Mercier-Delarue, S., Caro, V., Enouf, V., Simon, F., Molina, J., & van der Werf, S. (2012). I223R mutation in influenza A(H1N1)pdm09 neuraminidase confers reduced susceptibility to oseltamivir and Zanamivir and enhanced resistance with H275Y. PLoS One, 7, e37095.
Shanthi, V., Ramanathan, K., & Sethumadhavan, R. (2010). Exploring the role of C-H....pi interactions on the structural stability of single chain “all-alpha” proteins. Applied Biochemistry and Biotechnology, 160, 1473–1483.
Karthick, V., Shanthi, V., Rajasekaran, R., & Ramanathan, K. (2012). Exploring the cause of oseltamivir resistance against mutant H274Y neuraminidase by molecular simulation approach. Applied Biochemistry and Biotechnology, 167, 237–249.
Preethi, B., Shanthi, V., & Ramanathan, K. (2015). Investigation of nalidixic acid resistance mechanism in Salmonella enterica using molecular simulation techniques. Applied Biochemistry and Biotechnology, 177, 528–540.
Kumar, A., Shanthi, V., & Ramanathan, K. (2016). Discovery of potential ALK inhibitors by virtual screening approach. 3 Biotech, 6, 21.
Shoichet, B. K. (2004). Virtual screening of chemical libraries. Nature, 432, 862–865.
Karthick, V., Ramanathan, K., Shanthi, V., & Rajasekaran, R. (2013). Identification of potential inhibitors of H5N1 influenza A virus neuraminidase by ligand-based virtual screening approach. Cell Biochemistry and Biophysics, 66, 657–669.
Shahlaei, M., & Doosti, E. (2016). Virtual screening based on pharmacophore model followed by docking simulation studies in search of potential inhibitors for p38 map kinase. Biomedicine & Pharmacotherapy, 80, 352–372.
Kumar, S. P., & Jha, P. C. (2016). Multi-level structure-based pharmacophore 20ruids20g of caspase-3-non-peptide complexes: extracting essential pharmacophore features and its application to virtual screening. Chemico-Biological Interactions, 254, 207–220.
Tseng, T. S., Chuang, S. M., Hsiao, N. W., Chen, Y. W., Lee, Y. C., Lin, C. C., Huang, C., & Tsai, K. C. (2016). Discovery of a potent cyclooxygenase-2 inhibitor, S4, through docking-based pharmacophore screening, in vivo and in vitro estimations. Molecular BioSystems, 12, 2541–2551.
Khanfar, M. A., Al-Qtaishat, S., Habash, M., & Taha, M. O. (2016). Discovery of potent adenosine A2a antagonists as potential anti-Parkinson disease agents. Non-linear QSAR analyses integrated with pharmacophore modeling. Chemico-Biological Interactions, 254, 93–101.
Ronca, R., Giacomini, A., Di Salle, E., Coltrini, D., Pagano, K., Ragona, L., Matarazzo, S., Rezzola, S., Maiolo, D., Torrella, R., Moroni, E., Mazzieri, R., Escobar, G., Mor, M., Colombo, G., & Presta, M. (2015). Long-pentraxin 3 derivative as a small-molecule FGF trap for cancer therapy. Cancer Cell, 28, 225–239.
Bhadauriya, A., Dhoke, G. V., Gangwal, R. P., Damre, M. V., & Sangamwar, A. T. (2013). Identification of dual acetyl-CoA carboxylases 1 and 2 inhibitors by pharmacophore based virtual screening and molecular docking approach. Molecular Diversity, 17, 139–149.
Berman, H. M., Westbrook, J., Feng, Z., Gilliland, G., Bhat, T. N., & Weissig, H. (2000). The protein data bank. Nucleic Acids Research, 28, 235–242.
Vavricka, C. J., Li, Q., Wu, Y., Qi, J., Wang, M., Liu, Y., Gao, F., Liu, J., Feng, E., He, J., Wang, J., Liu, H., Jiang, H., & Gao, G. F. (2011). Structural and functional analysis of laninamivir and its octanoate prodrug reveals group specific mechanisms for influenza NA inhibition. PLoS Pathogens, 7, e1002249.
Irwin, J. J., & Shoichet, B. K. (2005). ZINC—a free database of commercially available compounds for virtual screening. Journal of Chemical Information and Modeling, 45, 177–182.
Chang, C. K., Jeyachandran, S., Hu, N. J., Liu, C. L., Lin, S. Y., Wang, Y. S., Chang, Y. M., & Hou, M. H. (2016). Structure-based virtual screening and experimental validation of the discovery of inhibitors targeted towards the human corona virus nucleocapsid protein. Molecular BioSystems, 12, 59–66.
Dixon, S. L., Smondyrev, A. M., & Rao, S. N. (2006). PHASE: a novel approach to pharmacophore modeling and 3D database searching. Chemical Biology & Drug Design, 67, 370–372.
Lipinski, C. A. (2000). Drug-like properties and the causes of poor solubility and poor permeability. Journal of Pharmacological and Toxicological Methods, 44, 235–249.
Huang, Q., Li, L. L., & Yang, S. Y. (2010). PhDD: a new pharmacophore-based de novo design method of drug-like molecules combined with assessment of synthetic accessibility. Journal of Molecular Graphics & Modelling, 28, 775–787.
Watts, K. S., Dalal, P., Murphy, R. B., Sherman, W., Friesner, R. A., & Shelley, J. C. (2010). ConfGen: a conformational search method for efficient generation of bioactive conformers. Journal of Chemical Information and Modeling, 50, 534–546.
Dixon, S. L., Smondyrev, A. M., Knoll, E. H., Rao, S. N., Shaw, D. E., & Friesner, R. A. (2006). PHASE: a new engine for pharmacophore perception, 3D QSAR model development and 3D database screening: 1. Methodology and preliminary results. Journal of Computer-Aided Molecular Design, 20, 647–671.
Bharatham, K., Bharatham, N., & Lee, K. W. (2007). Pharmacophore modeling for protein tyrosine phosphatase 1B inhibitors. Archives of Pharmacal Research, 30, 533–542.
Rajamanikandan, S., & Srinivasan, P. (2016). Pharmacophore modeling and structure-based virtual screening to identify potent inhibitors targeting LuxP of Vibrio harveyi. Journal of Receptor and Signal Transduction Research, 6, 1–16.
Singh, A. A., Sivakumar, D., & Somvanshi, P. (2011). Cataloguing functionally relevant polymorphisms in gene DNA ligase I: a computational approach. 3 Biotech, 1, 47.
Sastry, G. M., Adzhigirey, M., Day, T., Annabhimoju, R., & Sherman, W. (2013). Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. Journal of Computer-Aided Molecular Design, 27, 221–234.
Ekhteiari Salmas, R., Unlu, A., Bektaş, M., Yurtsever, M., Mestanoglu, M., & Durdagi, S. (2016). Virtual screening of small molecules databases for discovery of novel PARP-1 inhibitors: combination of in silico and in vitro studies. Journal of Biomolecular Structure & Dynamics, 17, 1–17.
Tikhonova, I. G., Sum, C. S., Neumann, S., Engel, S., Raaka, B. M., Costanzi, S., & Gershengorn, M. C. (2008). Discovery of novel agonists and antagonists of the free fatty acid receptor 1 (FFAR1) using virtual screening. Journal of Medicinal Chemistry, 51, 625–633.
Muralidharan, A. R., Selvaraj, C., Singh, S., Nelson Jesudasan, C. A., Geraldine, P., & Thomas, P. (2014). Virtual screening based on pharmacophoric features of known calpain inhibitors to identify potent inhibitors of calpain. Medicinal Chemistry Research, 23, 2445–2455.
Nair, S. B., Fayaz, S. M., & Krishnamurthy, R. G. (2012). In silico prediction of novel inhibitors of the DNA binding activity of FoxG1. Medicinal Chemistry, 8, 1155–1162.
Ramatenki, V., Dumpati, R., Vadija, R., Vellanki, S., Potlapally, S. R., Rondla, R., & Vuruputuri, U. (2017). Identification of new lead molecules against UBE2NL enzyme for cancer therapy. Applied Biochemistry and Biotechnology, 182, 1497–1517.
Ramezani, F., Amanlou, M., & Rafii-Tabar, H. (2014). Gold nanoparticle shape effects on human serum albumin corona interface: a molecular dynamic study. Journal of Nanoparticle Research, 16, 2512.
Schuttelkopf, A. W., & Van Aalten, D. M. F. (2004). PRODRG—a tool for high-throughput crystallography of protein–ligand complexes. Acta Crystallographica, 60, 1355–1363.
Karthick, V., Shanthi, V., Rajasekaran, R., & Ramanathan, K. (2013). In silico analysis of drug-resistant mutant of neuraminidase (N294S) against oseltamivir. Protoplasma, 250, 197–207.
Yang, S. Y. (2010). Pharmacophore modeling and applications in drug discovery: Challenges and recent advances. Drug Discovery Today, 15, 444–450.
Gubareva, L. V., Robinson, M. J., Bethell, R. C., & Webster, R. G. (1997). Catalytic and framework mutations in the neuraminidase active site of influenza viruses that are resistant to 4-guanidino-Neu5Ac2en. Journal of Virology, 71, 3385–3390.
Lipinski, C. A. (2004). Lead- and drug-like compounds: the rule-of-five revolution. Drug Discovery Today: Technologies, 1, 337–341.
Kalani, K., Yadav, D. K., Khan, F., Srivastava, S. K., & Suri, N. (2012). Pharmacophore, QSAR, and ADME based semi-synthesis and in vitro evaluation of ursolic acid analogsfor anticancer activity. Journal of Molecular Modeling, 18, 3389–3413.
Gaddaguti, V., Venkateswara Rao, T., & Prasada Rao, A. (2016). Potential mosquito repellent compounds of Ocimum species against 3N7H and 3Q8I of Anopheles Gambiae. 3 Biotech, 6, 26.
Sargsyan, K., Grauffel, C., & Lim, C. (2017). How molecular size impacts RMSD applications in molecular dynamics simulations. Journal of Chemical Theory and Computation, 13, 1518–1524.
Acknowledgements
The authors thank VIT University for providing “VIT SEED GRANT” for carrying out this research work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
K, R., V, S. Discovery of Potent Neuraminidase Inhibitors Using a Combination of Pharmacophore-Based Virtual Screening and Molecular Simulation Approach. Appl Biochem Biotechnol 184, 1421–1440 (2018). https://doi.org/10.1007/s12010-017-2625-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-017-2625-y