Abstract
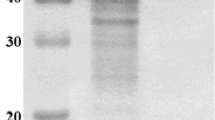
Streptomyces badius DB-1 produces α-amylase extracellularly, and its production was enhanced 5.1-fold (from 9.47 ± 0.51 to 48.23 ± 1.45 U mL−1) due to optimization by one-variable-at-a-time and statistical approaches. Soluble starch emerged as the most influential factor that strongly affected enzyme production. The purified enzyme is a monomer with a molecular mass of ~57 kDa and optimally active at 50 °C and pH 6.0. The enzyme hydrolyzes soluble as well as raw starches into simpler sugars with a high proportion (>40.0 %) of maltotetraose. It is optimally active at moderate temperature and generates maltooligosaccharides from starch, thus, useful as an antistale in bread making. It also plays a role in increasing the formation of maltooligosaccharides due to transglycosylation activity, thus, finds application in functional foods. This is the first report on the production of raw starch-digesting α-amylase by S. badius with transglycosylation activity.






Similar content being viewed by others
References
Barreteau, H., Delattre, C., & Michaud, P. (2006). Production of oligosaccharides as promising new food additive generation. Food Technology and Biotechnology, 44, 323–333.
Okada, M., Nakakuki, T. (1992). In F. W. Schenck (Ed.), Oligosaccharides: production, properties, and applications. New York: VHC Publishers.
Nakakuki, T. (2003). Development of functional oligosaccharides in Japan. Trends in Glycoscience and Glycotechnology, 15, 57–64.
Subramanian, G., Ayyadurai, S., Sharma, T., Singh, S. A., Rele, M., & Kumar, L. S. (2012). Studies on a maltohexaose (G6) producing alkaline amylase from a novel alkalophilic Streptomyces species. IIOABJ, 3, 15–30.
Agger, T., Spohr, A. B., & Nielsen, J. (2001). Alpha amylase production in high cell density submerged cultivation of Aspergillus oryzae and Aspergillus nidulans. Applied Microbiology and Biotechnology, 55, 81–84.
Chakraborty, S., Raut, G., Khopade, A., Mahadik, K., & Kokare, C. (2012). Study on calcium ion independent α-amylase from haloalkaliphilic marine Streptomyces strain A3. Indian Journal of Biotechnology, 11, 427–437.
Sharma, A., & Satyanarayana, T. (2010). High maltose-forming, Ca2+-independent and acid stable α-amylase from a novel acidophilic bacterium, Bacillus acidicola. Biotechnology Letters, 32, 1503–1507.
Lonsane, B. K., & Ramesh, M. V. (1990). Production of bacterial thermostable α-amylase by solid-state fermentation: a potential tool for achieving economy in enzyme production and starch hydrolysis. Advances in Applied Microbiology, 35, 1–56.
Sharma, T. K., Bhadane, V. A., Kumar, L. S., Rele, M. V., Bhawar, G., & Rahman, I. (2013). Optimization of the production of a maltooligosaccharides producing amylase from the alkalophilic Streptomyces lonarensis strain NCL 716 using SVR modeling. Starch, 65, 179–185.
Singh, R., Kapoor, V., & Kumar, V. (2011). Influence of carbon and nitrogen sources on the α-amylase production by a newly isolated thermophilic Streptomyces sp. MSC702 (MTCC 10772). Asian Journal of Biotechnology, 3, 540–553.
Syed, D. G., Agasar, D., & Pandey, A. (2009). Production and partial purification of α-amylase from a novel isolate Streptomyces gulbargensis. Journal of Industrial Microbiology and Biotechnology, 36, 189–194.
Kar, S., Ray, R. C., & Mohapatra, U. B. (2008). α-amylase production by Streptomyces erumpens MTCC 7317 in solid state fermentation using response surface methodology (RSM). Polish Journal of Microbiology, 57, 289–296.
Mouna imen, O., & Mahmoud, K. (2015). Statistical optimization of cultural conditions of an halophilic alpha-amylase production by halophilic Streptomyces sp. grown on orange waste powder. Biocatalysis and Agricultural Biotechnology, 4, 685–693.
Shabbiri, K., Adnan, A., Noor, B., & Jamil, S. (2012). Optimized production, purification and characterization of alpha amylase by Brevibacterium linens DSM 20158, using bio-statistical approach. Annals of Microbiology, 62, 523–532.
Wenster-Botz, D. (2000). Experimental design for fermentation media development: statistical design or global random search? Journal of Bioscience and Bioengineering, 90, 473–483.
Uma Maheswar Rao J. L., & Satyanarayana, T. (2007). Improving production of hyperthermostable and high maltose-forming a-amylase by an extreme thermophile Geobacillus thermoleovorans using response surface methodology and its applications. Bioresource Technology, 98, 345–352.
Primarini, D., & Ohta, Y. (2000). Some enzyme properties of raw starch digesting amylases from Streptomyces sp. no. 4. Starch, 52, 28–32.
Kaneko, T., Ohno, T., & Ohisa, N. (2005). Purification and characterization of a thermostable raw starch digesting amylase from a Streptomyces sp. isolated in a milling factory. Bioscience, Biotechnology, and Biochemistry, 69, 1073–1081.
Yang, C. H., & Liu, W. H. (2004). Purification and properties of a maltotriose-producing α-amylase from Thermobifida fusca. Enzyme and Microbial Technology, 35, 254–260.
Kamon, M., Sumitani, J., Tani, S., Kawaguchi, T., Kamon, M., Sumitani, J., et al. (2015). Characterization and gene cloning of a maltotriose-forming exo-amylase from Kitasatospora sp. MK-1785. Applied Microbiology and Biotechnology, 99, 4743–4753.
Park, K. H. (2012). Biotechnological modification of bioactive natural compounds for food industry. Journal of Food and Drug Analysis, 20, 189–193.
Kumar, P., & Satyanarayana, T. (2008). Potential applications of microbial enzymes in improving quality and shelf-life of bakery products. In A. Koutinas, A. Pandey, & C. Larroche (Eds.), Bioprocesses in food industry (pp. 132–146). New Delhi: Asiatech Publishers Inc.
Miller, G. L. (1959). Use of dinitrosalicyclic acid reagent for determination of reducing sugar. Analytical Chemistry, 31, 426–429.
Bradford, M. M. (1976). A rapid and sensitive method for quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry, 72, 248–254.
Tripathi, G., & Rawal, S. K. (1998). Simple and efficient protocol for isolation of high molecular weight DNA from Streptomyces aureofaciens. Biotechnology Techniques, 12, 629–631.
Laemmli, U. K. (1970). Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature, 227, 680–685.
Nisha, M., & Satyanarayana, T. (2013). Characterization of recombinant amylopullulanase (gt-apu) and truncated amylopullulanase (gt-apuT) of the extreme thermophile Geobacillus thermoleovorans NP33 and their action in starch saccharification. Applied Microbiology and Biotechnology, 97, 6279–6292.
Hansen, S. A. (1975). Thin layer chromatographic method for identification of oligosaccharides in starch hydrolysates. Journal of Chromatography, 105, 388–390.
Mishra, R., & Maheshwari, R. (1996). Amylases of the thermophilic fungus Thermomyces lanuginosus; their purification, properties, action on starch and response to heat. Journal of Bioscience, 21, 653–672.
Phillips, L. (1992). The distribution of phenotypic and genotypic characters within Streptomycetes and their relationship to antibiotic production. PhD thesis, University of Warwick.
Saddler, G. S., O’donnell, A. G., Goodfellow, M., & Minnikin, D. E. (1987). Pattern recognition in the analysis of Streptomycete fatty acids. Journal of General Microbiology, 133, 1137–1147.
Sujarit, K., Kudo, T., Ohkuma, M., Pathom-Aree, W., & Lumyong, S. (2016). Streptomyces Palmae sp. nov., isolated from oil palm (Elaeis guineensis) rhizosphere soil. International Journal of Systematic and Evolutionary Microbiology. doi:10.1099/ijsem.0.001298.
Krishnakumar, S., Bai, V. D. M., & Premkumar, J. (2015). Production of alpha amylase by salt-tolerant actinomycete Streptomyces sp.-SBU3 isolated from marine sponge. Indian Journal of Geo-Marine Sciences, 44, 1–6.
Naragani, K., Muvva, V., Munaganti, R. K., & Heema Bindu, B. S. S. N. (2015). Studies on optimization of amylase production by Streptomyces cheonanensis VUK-A isolated from mangrove habitats. Journal of Advanced Biology and Biotechnology, 3, 165–172.
Mazotto, A. M., Cedrola, S. M. L., Lins, U., Rosado, A. S., Silva, K. T., Chaves, J. Q., et al. (2010). Keratinolytic activity of Bacillus subtilis AMR using human hair. Letters in Applied Microbiology, 50, 89–96.
Narayana, K. J. P., & Vijayalakshmi, M. (2008). Production of extracelluar α-amylase by Streptomyces albidoflavus. Asian Journal of Biochemistry, 3, 194–197.
Poornima, R., Sahu, M. K., Sivakumar, K., & Pushpavalli, V. (2008). Optimization of α-amylase production by actinomycetes strain AE-19 isolated from shrimp pond. Trends in Applied Science Research, 3, 45–52.
Zhu, W., Dongmei, C., Gugue, C., Peng, Q., & Shen, P. (2007). Purification and characterization of a thermostable protease from a newly isolated Geobacillus sp. YMTC 1049. Enzyme and Microbial Technology, 40, 1592–1597.
Kammoun, R., Naili, B., & Bejar, S. (2008). Application of a statistical design to the optimization of parameters and culture medium for α-amylase production by Aspergillus oryzae CBS 819.72 grown on gruel (wheat grinding by-product). Bioresource Technology, 99, 5602–5609.
Gangadharan, D., Sivaramakrishnan, S., Nampoothiri, K. M., Sukumaran, R. K., & Pandey, A. (2008). Response surface methodology for the optimization of α-amylase production by Bacillus amyloliquefaciens. Bioresource Technology, 99, 4597–4602.
Hashemi, M., Razavi, S. H., Shojaosadati, S. A., Mousavi, S. M., Khajeh, K., & Safari, M. (2010). Development of a solid-state fermentation process for production of an alpha amylase with potentially interesting properties. Journal of Bioscience and Bioengineering, 110, 333–337.
Kumar, V., & Satyanarayana, T. (2014). Production of thermo-alkali-stable xylanase by a novel polyextremophilic Bacillus halodurans TSEV1 in cane molasses medium and its applicability in making whole wheat bread. Bioprocess and Biosystems Engineering, 37, 1043–1053.
McMahon, H. E. M., Kelly, C. T., & Fogarty, W. M. (1998). High maltose-producing amylolytic system of a Streptomyces sp. Biotechnology Letters, 21, 23–26.
Goldberg, J. D., & Edwards, C. (1990). Purification and characterization of an extracellular amylase from a thermophilic streptomycete. Journal of Applied Bacteriology, 69, 712–717.
Takasaki, Y., Kitajima, M., Tsuruta, T., Nonoghchi, M., Hayashi, S., & Imada, K. (1991). Maltotriose-producing amylase from Microbacterium imperiale. Agricultural and Biological Chemistry, 55, 687–692.
Mehta, D., & Satyanarayana, T. (2013). Dimerization mediates thermo-adaptation, substrate affinity and transglycosylation in a highly thermostable maltogenic amylase of Geobacillus thermoleovorans. PloS One, 8, e73612.
Nisha, M., & Satyanarayana, T. (2014). Characterization and multiple applications of a highly thermostable and Ca2+-independent amylopullulanase of the extreme thermophile Geobacillus thermoleovorans. Applied Biochemistry and Biotechnology, 174, 2594–2615.
Martin, M. L., & Hoseney, R. C. (1991). A mechanism of bread firming. II. Role of starch hydrolyzing enzymes. Cereal Chemistry, 68, 503–507.
Acknowledgments
The authors gratefully acknowledge financial assistance from the Department of Biotechnology and University Grants Commission, Government of India, New Delhi while carrying out the work presented in the manuscript. The authors thank Dr. Debananda Ningthoujam, Department of Biochemistry, Manipur University, Imphal (India) for providing actinobacterial cultures. The authors wish to thank Mr. Vijay Kumar Gupta (Tushar Nutritive Food Industry, New Delhi) for extending help in assessing the applicability of amylase in bread making.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
ESM 1
(DOC 1885 kb)
Rights and permissions
About this article
Cite this article
Shivlata, L., Satyanarayana, T. Characteristics of Raw Starch-Digesting α-Amylase of Streptomyces badius DB-1 with Transglycosylation Activity and Its Applications. Appl Biochem Biotechnol 181, 1283–1303 (2017). https://doi.org/10.1007/s12010-016-2284-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-016-2284-4