Abstract
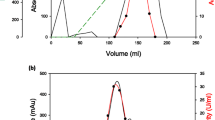
A thermophilic fungus Thermomyces lanuginous strain IISc 91, secreted one form each of α-amylase and glucoamylase during growth. Both enzymes were purified to homogeneity by ion-exchange and gel-filtration chromatography and obtained in mg quantities. α-Amylase was considered to be a dimeric protein of ∼ 42 kDa and contained 5% (by mass) carbohydrate. It was maximally active at pH 5.6 and at 65°C. It had an activation energy of 44 kJ mol-1. The apparent Km for soluble starch was 2.5 mg ml-1. The enzyme produced exceptionally high levels of maltose from raw potato starch. At 50°C, the enzyme was stable for > 7h. At 65°C, α-amylase was nearly 8-times more stable in the presence of calcium. Addition of calcium increaed the melting temperature of α-amylase from 66°C to 73°C. Upon incubation at 94°C, α-amylase was progressively and irreversibly inactivated, and converted into an inactive 72 kDa trimeric species.
Glucoamylase was a monomeric glycoprotein of ∼ 45 kDa with a carbohydrate content of 11% (by mass). It effected up to 76% conversion of starch in 24 h producing glucose as the sole product. Its apparent Km for soluble starch was 0.04 mg ml-1 and Vmax was 660 Mmol glucose min-1 mg protein-1. It also hydrolyzed maltose. Its activity on maltooligosaccharides increased with the chain length of the substrates. Glucoamylase was stable at 60°C for over 7h. Its activation energy was 61 kJ mol-1 Glucoamylase did not show synergistic effect with α-amylase. The properties of α-amylase and glucoamylase of Thermomyces lanuginosus strain IISc 91 suggest their usefulness in the commercial production of maltose and glucose syrups.
Similar content being viewed by others
References
Abe J, Nakajima K, Nagao H, Hizukuri S and Obata K 1988 Properties of the raw-starch digesting amylase ofAspergillus sp. K-27: A synergistic action of glucoamylase and α-amylase;Carbahydr. Res. 175 85–92
Anand L, Krishnamurthy S and Vithayathil P J 1990 Purification and properties of xylanase from the thermophilic fungus,Humicola lanuginosa (Griffon and Maublanc) Bunce;Arch. Biochem, Biophys. 276 546–553
Bealin-Kelly F, Kelly A. T and Fogarty W M 1991 Studies on the thennostability of the α-amylase ofBacillus caldovelox;Appl. Microbial. Biotechnol. 36 332–336
Bencze W L and Schmid K 1957 Determination of tyrosine and tryptophan in proteins;Anal. Chem. 29 1193–1196
Doyle E M, Kelly A. T and Fogarty W M 1989 The high maltose producing α-amylase ofPenicillium expansum;Appl. Microbiol. Biotechnol. 30 492–496
Dubois M, Gilles K A, Hamilton J K, Rebers P A and Smith F 1956 Colorimetric method for determination of sugars and related substrates;Anal. Chem. 28 350–356
Fagerstrom R, Vainio A, Suoranta K, Pakula T, Kalkkinen N and Tarkkeli H 1990 Comparison of two glucoamylases fromHormoconis resinae;J Gen. Microbiol. 136 913–920
Ferguson K A 1964 Starch gel electrophoresis; application to the classification of pituitary proteins and polypeptides;Metab. Clin. Exp. 13 985–1002
Hayashida S, Nakaahava K, Kuroda K, Kamachi T, Ohta K, Iwanaga S, Miyata T and Sakayi Y 1988 Evidence for post translational generation of multiple forms of Aspergillus awamori glucoamylase;Agric. Biol. Chem.,52 273–275
Hedrick J L and Smith A J 1968 Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis;Arch. Biochem. Biophys. 126 155–164
Jensen B, Olsen J and Allerman K 1988 Purification of extracellular amylolytic enzymes from the thermophilic fungusThermomyces lanuginosus;Can. J. Microbiol. 34 218–223
Jensen B and Olsen J 1992 Physicochemical properties of a purified α-amylase from the thermophilic fungusThermomyces lanuginosus;Enzyme Microb. Technol. 14 112–116
Johnson E J 1979 Thermophile genetics and the genetic determinants of thermophily; inStrategies of microbial life in extreme environments (ed.) M Shilo (Weinheim: Verlag Chemie) pp 471–487
Khandke K M, Vithayathil P J and Krishnamurthy S 1989 Purification of xylanase, β-glucosidase, endocellulase and exocellulase from a thermophilic fungusThermoascus aurantiacus;Arch. Biochem. Biophys. 274 491–500
Koch R, Spreinat A, Lemke K and Antranikian G 1991 Purification and properties of a hyperthermoactive α-amylase from the archaebacteriumPyrococcus woesei;Arch. Microbiol. 155 572–578
Laderman K A, Davis B R, Krutzch H C, Lewis M, Griko Y V, Privalov P L and Anfinsen A. B 1994 The purification and characterization of an extremely thermostable α-amylase from the hyperthermophilicarchaebacterium Pyrococcus furiosus;J. Biol. Chem. 268 24394–24401
Laemmli U K 1970 Cleavage of structural proteins during the assembly of the head of bacteriophage T4;Nature (London) 227 680–685
Lowry O H, Rosebrough N J, Farr A L and Randall R J 1951 Protein measurements with the Folin phenol reagent;J. Biol. Chem. 193 265–275
Lugtenberg B, Meijers J, Peters R, van der Hoek P and van Alphen L 1975 Electrophoretic resolution of the major outer membrane protein ofEscherichia coli K12 into four bands;FEBS Lett. 58 254–258
McComb R B and Yushok W D 1957 Colorimetric estimation of D-glucose and 2-deoxy D-glucose with glucose oxidase;J. Franklin Inst. 265 417–422
Moore S and Stein W H 1963 Chromatographic determination of amino acids by the use of automatic recording equipment;Methods Enzymol. 6 819–831
Ono K, Shintani K, Shigeta S and Oka S 1988 Various molecular species in glucoamylase fromAspergillus niger;Agric. Biol. Chem. 52 1689–1698
Rao V B, Sastri N V S and Subba Rao P V 1981 Purification and characterization of a thermostable glucoamylase from the thermophilic fungusThermomyces lanuginosus;Biochem. J. 193 379–387
Razzaque A and Ueda S 1978 Glucoamylaseof Aspergillus oryzae;J. Ferment. Technol. 56 296–302
Robyt J F and Ackerman R J 1973 Structure and function of amylases. II. Multple forms ofBacillus subtilis α-amylase;Arch. Biochem. Biophys. 156 445–451
Somogyi M 1952 Notes on sugar determination;J. Biol. Chem. 195 19–23
Svensson B, Pedersen T G, Svendsen I B, Sakai T and Ottesen M 1982 Characterization of two forms of glucoamylase fromAspergillus niger;Carlsberg Res. Commun. 47 55–69
Takahashi T, Tsuchida Y and Irie M 1978 Purification and some properties of three forms of glucoamylase fromRhizopus species;J. Biochem. 84 1183–1194
Taylor P M, Napier E J and Fleming I D 1978 Some properties of a glucoamylase produced by the thermophilic fungusHumicola lanuginosa;Carbohydr. Res. 61 301–308
Trevelyan W E, Procter D P and Harrison J S 1950 Detection of sugars on paper chromatograms;Nature (London) 166 444–445
van Brunt J 1986 Fungi: The perfect hosts?;Biotechnology 4 1057–1062
Vihinen M and Mantsala R 1989 Microbial amylolytic enzymes;CRC Crit. Rev. Biochem. Mol. Biol. 241 329–418
Vogel H L 1964 Distribution of lysine pathways among fungi: Evolutionary implications;Am. Nat. 98 435–446
Yamasaki Y, Suzuki Y and Ozawa J 1977 Purification and properties of two forms of glucoamylase fromPenicillium oxalicum;Agric. Biol. Chem. 41 755–762
Yemm E W and Willis A J 1954 The estimation of carbohydrates in plant extracts by anthrone;Biochem.J. 57 508–514
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Mishra, R.S., Maheshwari, R. Amylases of the thermophilic fungusThermomyces lanuginosus: Their purification, properties, action on starch and response to heat. J Biosci 21, 653–672 (1996). https://doi.org/10.1007/BF02703143
Received:
Issue Date:
DOI: https://doi.org/10.1007/BF02703143