Abstract
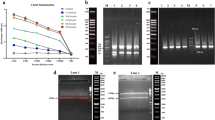
Leukemic cancer stem cells (LSCs), as a unique cell population in acute myeloid leukemia (AML) marked by CD123 overexpression, are thought to play a key role in relapsed AML after chemotherapy. Thus, CD123 is considered as a particularly important target candidate for antibody-derived diagnosis and therapy. In the present work, we constructed an immunized murine antibody phage display library and isolated the functional anti-CD123 Single-chain fragment variable (scFv) clones. We also introduced fusing variable light (VL) and heavy (VH) chains with a new 18-amino acid residue linker as an alternative to conventional linkers. CD123-specific phage clones were progressively enriched through 4 rounds of biopanning, validated by phage ELISA, and anti-CD123 scFv clones with highest affinity were produced in Escherichia coli. The expression and purification of soluble scFv were verified by Western blot, and the results were indicative of the functionality of our proposed linker. The purified scFv specifically recognized CD123 by ELISA and flow cytometry, without any cross-reactivity with other related cell markers. Affinity of anti-CD123 scFv was measured to be 6.9 × 10−7 M, using the competitive ELISA. Our work, therefore, provides a framework for future studies involving biological functions and applications of our anti-CD123 scFv. It also reveals the feasibility of high throughput methods to isolate biomarker-specific scFvs.





Similar content being viewed by others
Abbreviations
- AML:
-
acute myeloid leukemia
- CD123:
-
cluster of differentiation 123
- LSCs:
-
leukemic cancer stem cells
- scFv:
-
single-chain fragment variable
- VH:
-
variable heavy chains of an antibody
- VL:
-
variable light chains of an antibody
References
Rubnitz, J. E., Gibson, B., & Smith, F. O. (2010). Acute myeloid leukemia. Hematology/Oncology Clinics of North America, 24(1), 35–63.
Jordan, C., et al. (2000). The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia, 14(10), 1777–1784.
Testa, U., et al. (2004). Interleukin-3 receptor in acute leukemia. Leukemia, 18(2), 219–226.
Ravandi, F., & Estrov, Z. (2006). Eradication of leukemia stem cells as a new goal of therapy in leukemia. Clinical Cancer Research, 12(2), 340–344.
Liu, K., et al. (2015). CD123 and its potential clinical application in leukemias. Life Sciences, 122, 59–64.
Taussig, D. C., et al. (2005). Hematopoietic stem cells express multiple myeloid markers: implications for the origin and targeted therapy of acute myeloid leukemia. Blood, 106(13), 4086–4092.
Hammers, C. M., & Stanley, J. R. (2014). Antibody phage display: technique and applications. The Journal of Investigative Dermatology, 134(2), e17.
Sidhu, S. S. (2000). Phage display in pharmaceutical biotechnology. Current Opinion in Biotechnology, 11(6), 610–616.
Bird, R. E., et al. (1988). Single-chain antigen-binding proteins. Science, 242(4877), 423–426.
Carter, P. J. (2006). Potent antibody therapeutics by design. Nature Reviews Immunology, 6(5), 343–357.
Pansri, P., et al. (2009). A compact phage display human scFv library for selection of antibodies to a wide variety of antigens. BMC Biotechnology, 9(1), 6.
Stein, C., et al. (2010). Novel conjugates of single‐chain Fv antibody fragments specific for stem cell antigen CD123 mediate potent death of acute myeloid leukaemia cells. British Journal of Haematology, 148(6), 879–889.
Gu, X., et al. (2010). Molecular modeling and affinity determination of scFv antibody: proper linker peptide enhances its activity. Annals of Biomedical Engineering, 38(2), 537–549.
Alfthan, K., et al. (1995). Properties of a single-chain antibody containing different linker peptides. Protein Engineering, 8(7), 725–731.
Shan, D., et al. (1999). Characterization of scFv-Ig constructs generated from the anti-CD20 mAb 1F5 using linker peptides of varying lengths. The Journal of Immunology, 162(11), 6589–6595.
Breitling, F., et al. (1991). A surface expression vector for antibody screening. Gene, 104(2), 147–153.
Barbas, C. F. (2001). Phage display: a laboratory manual. New York: Cold Spring Harbor Laboratory Press.
Lee, C. M., et al. (2007). Selection of human antibody fragments by phage display. Nature Protocols, 2(11), 3001–3008.
Martineau, P. (2010). Affinity measurements by competition ELISA. In Antibody engineering Vol 2 (pp. 657–665). Berlin Heidelberg: Springer.
Roncolato, E. C., et al. (2015). Phage display as a novel promising antivenom therapy: a review. Toxicon, 93, 79–84.
Kellner, C., Nodehi, S. M., Peipp, M. (2010) Mouse immune libraries for the generation of ScFv fragments directed against human cell surface antigens. In Antibody engineering Vol 1 (pp. 47–63). Berlin Heidelberg: Springer.
Hussein, A. H., et al. (2007). Construction and characterization of single-chain variable fragment antibodies directed against the Bordetella pertussis surface adhesins filamentous hemagglutinin and pertactin. Infection and Immunity, 75(11), 5476–5482.
Dong, L., et al. (2003). Generation of affinity matured scFv antibodies against mouse neural cell adhesion molecule L1 by phage display. Biochemical and Biophysical Research Communications, 301(1), 60–70.
Wang, N., et al. (2008). Construction and characterization of phage display library: recognition of mouse serologically detected male (SDM) antigen. Animal Reproduction Science, 104(1), 93–110.
Sepulveda, J., & Shoemaker, C. B. (2008). Design and testing of PCR primers for the construction of scFv libraries representing the immunoglobulin repertoire of rats. Journal of Immunological Methods, 332(1), 92–102.
Nishi, M., et al. (2014). Ligation-based assembly for constructing mouse synthetic scFv libraries by chain shuffling with in vivo-amplified V H and V L fragments. Journal of Immunological Methods, 412, 53–69.
Shahsavarian, M. A., et al. (2014). Exploitation of rolling circle amplification for the construction of large phage-display antibody libraries. Journal of Immunological Methods, 407, 26–34.
Shi, J., et al. (2015). Expression, purification, and characterization of scar tissue neovasculature endothelial cell-targeted rhIL10 in Escherichia coli. Applied Biochemistry and Biotechnology, 175(1), 625–634.
Sonoda, H., et al. (2011). Cytoplasmic production of soluble and functional single-chain Fv-Fc fusion protein in Escherichia coli. Biochemical Engineering Journal, 53(3), 253–259.
Miethe, S., et al. (2013). Production of single chain fragment variable (scFv) antibodies in Escherichia coli using the LEX™ bioreactor. Journal of Biotechnology, 163(2), 105–111.
Sun, Y., et al. (2015). Expression and characterization of the extracellular domain of human HER2 from escherichia coli, and production of polyclonal antibodies against the recombinant proteins. Applied Biochemistry and Biotechnology, 176(4), 1029–1043.
Turner, D. J., Ritter, M. A., & George, A. J. (1997). Importance of the linker in expression of single-chain Fv antibody fragments: optimisation of peptide sequence using phage display technology. Journal of Immunological Methods, 205(1), 43–54.
Wang, S.-H., et al. (2006). Construction of single chain variable fragment (scFv) and BiscFv-alkaline phosphatase fusion protein for detection of Bacillus anthracis. Analytical Chemistry, 78(4), 997–1004.
Klement, M., et al. (2015). Effect of linker flexibility and length on the functionality of a cytotoxic engineered antibody fragment. Journal of Biotechnology, 199, 90–97.
Chen, X., Zaro, J. L., & Shen, W.-C. (2013). Fusion protein linkers: property, design and functionality. Advanced Drug Delivery Reviews, 65(10), 1357–1369.
Schmiedl, A., Breitling, F., & Dübel, S. (2000). Expression of a bispecific dsFv–dsFv’ antibody fragment in Escherichia coli. Protein Engineering, 13(10), 725–734.
Atwell, J. L., et al. (1996). Design and expression of a stable bispecific scFv dimer with affinity for both glycophorin and N9 neuraminidase. Molecular Immunology, 33(17), 1301–1312.
Feng, J., et al. (2003). Design and assembly of anti-CD16 scFv antibody with two different linker peptides. Journal of Immunological Methods, 282(1), 33–43.
Le Gall, F., et al. (2004). Effect of linker sequences between the antibody variable domains on the formation, stability and biological activity of a bispecific tandem diabody. Protein Engineering Design and Selection, 17(4), 357–366.
Albrecht, H., DeNardo, G. L., & DeNardo, S. J. (2006). Monospecific bivalent scFv-SH: effects of linker length and location of an engineered cysteine on production, antigen binding activity and free SH accessibility. Journal of Immunological Methods, 310(1), 100–116.
Gustavsson, M., et al. (2001). Stable linker peptides for a cellulose-binding domain–lipase fusion protein expressed in Pichia pastoris. Protein Engineering, 14(9), 711–715.
Kumada, Y., et al. (2007). Polypeptide linkers suitable for the efficient production of dimeric scFv in Escherichia coli. Biochemical Engineering Journal, 35(2), 158–165.
Whitlow, M., et al. (1993). An improved linker for single-chain Fv with reduced aggregation and enhanced proteolytic stability. Protein Engineering, 6(8), 989–995.
Zhang, J., et al. (2009). Design and optimization of a linker for fusion protein construction. Progress in Natural Science, 19(10), 1197–1200.
Kiss, M. M., et al. (2011). Phage ESCape: an emulsion-based approach for the selection of recombinant phage display antibodies. Journal of Immunological Methods, 367(1), 17–26.
Alirezapour, B., et al. (2013). Production and characterization of recombinant scFv against digoxin by phage display technology. Monoclonal Antibodies in Immunodiagnosis and Immunotherapy, 32(3), 172–179.
Min, W.-K., et al. (2010). Production and characterization of monoclonal antibody and its recombinant single chain variable fragment specific for a food-born mycotoxin, fumonisin B1. Bioprocess and Biosystems Engineering, 33(1), 109–115.
Martsev, S. P., et al. (1998). Antiferritin single-chain antibody: a functional protein with incomplete folding? FEBS Letters, 441(3), 458–462.
Drees, J. J., et al. (2014). Soluble production of a biologically active single-chain antibody against murine PD-L1 in Escherichia coli. Protein Expression and Purification, 94, 60–66.
Schaefer, J. V., & Pluckthun, A. (2012). Transfer of engineered biophysical properties between different antibody formats and expression systems. Protein Engineering Design and Selection, 25(10), 485–506.
Renaut, L., et al. (2012) Affinity maturation of antibodies: optimized methods to generate high-quality ScFv libraries and isolate IgG candidates by high-throughput screening. In Antibody engineering Vol 1 (pp. 451–461). Berlin Heidelberg: Springer.
Xia, J., et al. (2013). Isolation, identification and expression of specific human CD133 antibodies. Scientific Reports, 3, 3320.
Blazek, D., et al. (2004). Generation and characterization of single-chain antibody fragments specific against transmembrane envelope glycoprotein gp46 of maedi-visna virus. Journal of Virological Methods, 115(1), 83–92.
Kim, H. S., et al. (2011). Improvement of anti-Burkholderia mouse monoclonal antibody from various phage-displayed single-chain antibody libraries. Journal of Immunological Methods, 372(1–2), 146–161.
Boder, E. T., Midelfort, K. S., & Wittrup, K. D. (2000). Directed evolution of antibody fragments with monovalent femtomolar antigen-binding affinity. Proceedings of the National Academy of Sciences, 97(20), 10701–10705.
Hudson, P. J., & Kortt, A. A. (1999). High avidity scFv multimers: diabodies and triabodies. Journal of Immunological Methods, 231(1), 177–189.
Power, B. E., & Hudson, P. J. (2000). Synthesis of high avidity antibody fragments (scFv multimers) for cancer imaging. Journal of Immunological Methods, 242(1), 193–204.
Acknowledgments
This work was supported by Postgraduate Office, Pasteur Institute of Iran, Tehran, Iran.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Moradi-Kalbolandi, S., Davani, D., Golkar, M. et al. Soluble Expression and Characterization of a New scFv Directed to Human CD123. Appl Biochem Biotechnol 178, 1390–1406 (2016). https://doi.org/10.1007/s12010-015-1954-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12010-015-1954-y